Executive Summary
In the high-stakes domain of Assisted Reproductive Technology (ART), the selection of the most viable embryo remains the singular bottleneck preventing the standardization of single-embryo transfer (SET) and the optimization of time-to-pregnancy (TTP). Traditional morphological grading, while foundational, is subjective and static, failing to capture the dynamic physiological state of the developing blastocyst. Invasive Preimplantation Genetic Testing for Aneuploidy (PGT-A), though accurate for genomic integrity, imposes biopsy-related stress and costs that limit universal application.
This clinical deep-dive analyzes METAPHOR (Metabolic Evaluation through Phasor-based Hyperspectral Imaging and Organelle Recognition), a paradigm-shifting technology that merges multiphoton fluorescence lifetime imaging (FLIM) with artificial intelligence. By quantifying the intrinsic autofluorescence of metabolic coenzymes NADH and FAD, METAPHOR provides a non-invasive, label-free window into embryo bioenergetics. This analysis explores the molecular mechanisms of mitochondrial function, the engineering principles of phasor-based hyperspectral imaging, and the economic imperative for ART clinics to adopt metabolic profiling as the new gold standard for embryo selection.
Problem Statement: The “Black Box” of Embryo Viability
Clinical Perspective: The Morphology Trap
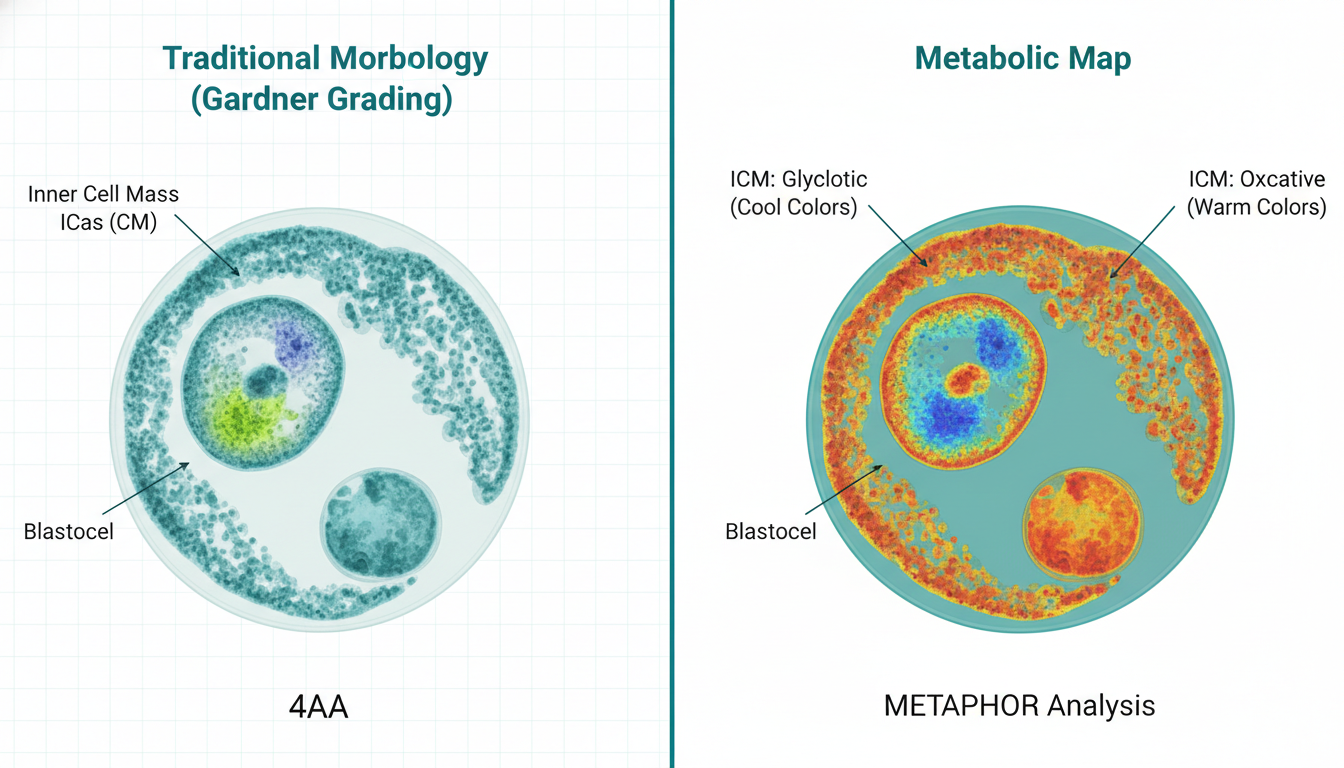
For clinical embryologists, the current standard of care—Gardner grading—is akin to judging an engine’s performance by looking at the car’s paint job. An embryo with a “AA” grade (fully expanded, tightly packed inner cell mass, cohesive trophectoderm) can still harbor lethal metabolic defects or aneuploidy. The static nature of brightfield microscopy misses the rate of development and the quality of cellular respiration. Clinicians are forced to gamble on “quiet” metabolism theories without direct measurement, leading to repeated implantation failures (RIF) and emotional exhaustion for patients.
Laboratory Perspective: The Biopsy Burden
The alternative, PGT-A, requires the laser-assisted removal of trophectoderm cells. While robust for assessing chromosomal copy number, this procedure is technically demanding, requires expensive sequencing infrastructure (often outsourced, delaying transfer), and inherently risks damaging the embryo. Furthermore, PGT-A does not assess mitochondrial function. A euploid embryo with insufficient ATP production capacity will fail to implant just as surely as an aneuploid one. There exists a critical diagnostic gap: a need for a functional, non-invasive assay that reads the embryo’s metabolic “vital signs” in real-time.
Theoretical Framework: Molecular Bioenergetics of the Blastocyst
The Metabolic Switch and Viability
The preimplantation embryo undergoes a profound metabolic metamorphosis. During the cleavage stage (Day 1-3), the embryo is metabolically “quiet,” relying primarily on pyruvate and lactate via oxidative phosphorylation (OXPHOS) to maintain basal homeostasis. As the embryo compacts and cavitates to form a blastocyst (Day 4-5), energy demand surges to support the Na+/K+ ATPase pumps required for blastocoel expansion.
At this critical juncture, a viable embryo must successfully transition to a glucose-based metabolism, engaging both glycolysis and OXPHOS. This “metabolic switch” is the definitive biomarker of developmental competence.
- High Viability Profile: A balance of efficient OXPHOS in the trophectoderm (TE) to fuel invasion, and regulated glycolysis in the Inner Cell Mass (ICM) to preserve pluripotency.
- Low Viability Profile: Mitochondrial dysfunction leads to a compensatory upregulation of glycolysis (Warburg-like effect) or “stressed” OXPHOS, generating reactive oxygen species (ROS) and altering the redox state.
Endogenous Fluorophores: NADH and FAD
METAPHOR exploits the intrinsic fluorescence of two key coenzymes in these pathways:
1. NADH (Nicotinamide Adenine Dinucleotide): Fluorescent in its reduced form. Its fluorescence lifetime significantly shortens when free in the cytosol (glycolysis) compared to when bound to enzymes in the electron transport chain (OXPHOS).
2. FAD (Flavin Adenine Dinucleotide): Fluorescent in its oxidized form. Its distribution allows for the direct visualization of mitochondrial networks.
By measuring the ratio of Free-to-Bound NADH and the optical redox ratio (NADH/FAD), we can derive a quantitative “metabolic trajectory” for each embryo.
Device Technology & Engineering: Phasor-Based Hyperspectral Imaging
Multiphoton Excitation and Safety
Unlike UV-based excitation which is cytotoxic to DNA, METAPHOR utilizes Two-Photon Excitation (2PE). A femtosecond pulsed laser (typically Ti:Sapphire, tunable around 780 nm) delivers high photon density only at the focal volume. This allows for deep tissue penetration with minimal phototoxicity and photobleaching, preserving embryo integrity for transfer.
The Phasor Approach: Geometry over Curve Fitting
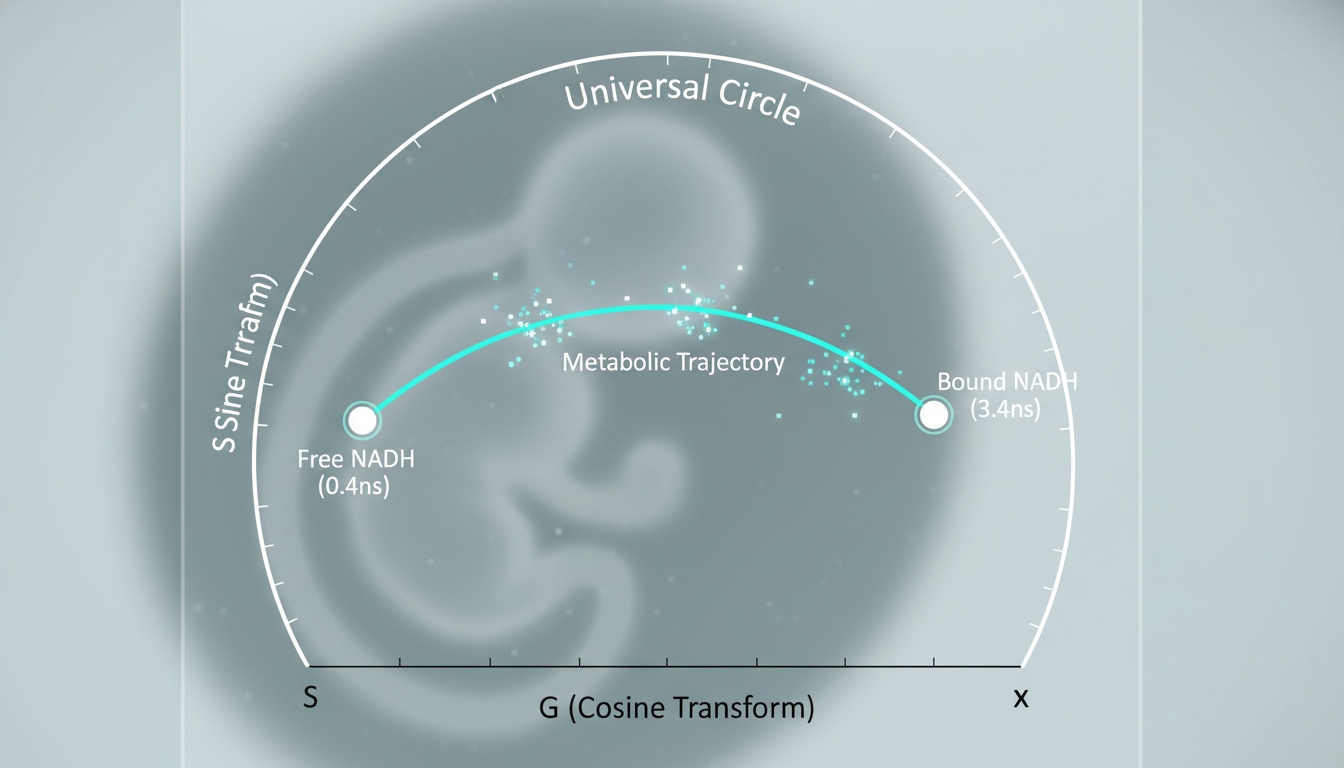
Traditional FLIM analysis relies on multi-exponential curve fitting of fluorescence decay, which is computationally expensive and prone to error in low-photon conditions (common in live embryos). METAPHOR employs the Phasor Approach, a coordinate-transformation technique:
1. Fourier Transform: The fluorescence decay at each pixel is Fourier transformed into a vector.
2. The Phasor Plot: This vector is plotted on a 2D semi-circle (the “universal circle”).
- Points on the circle represent single-exponential lifetimes.
- Points inside the circle represent complex mixtures.
3. Metabolic Mapping:
- Free NADH (Glycolytic signature) maps to a specific coordinate (short lifetime, ~0.4 ns).
- Bound NADH (OXPHOS signature) maps to a different coordinate (long lifetime, ~3.4 ns).
The position of each pixel on the line connecting these two poles directly correlates to the metabolic state of that specific cellular region. This allows the system to generate a pixel-by-pixel “metabolic map” of the embryo without requiring complex mathematical fitting.
Hyperspectral Data Cube & Organelle Recognition
Beyond lifetime, the system captures a hyperspectral data cube (x, y, λ). The spectral emission is split into typically 32 channels. This spectral resolution allows the AI to distinguish overlapping fluorophores (e.g., distinguishing NADH from NADPH or retinoids) and effectively segment the image.
The “Organelle Recognition” component utilizes this rich feature set to perform semantic segmentation of mitochondria. By analyzing the texture and distribution of the FAD signal, the AI calculates mitochondrial clustering metrics. Perinuclear mitochondrial clustering is often a sign of high developmental potential, whereas diffuse or aggregated peripheral mitochondria may signal stress.
Clinical Efficacy and AI Validation
Recent validation studies published in journals like PNAS demonstrate the superiority of METAPHOR over human grading:
- Sensitivity: The system distinguishes between euploid and aneuploid metabolic signatures with an Area Under the Curve (AUC) approaching 0.96, significantly higher than the ~0.65 AUC typical of morphological grading.
- Oocyte Assessment: The technology is equally applicable to oocytes, predicting blastulation potential based on the metabolic signature of the ooplasm before fertilization occurs—a capability currently non-existent in clinical practice.
- AI Classifier: The underlying algorithm (typically a Convolutional Neural Network or Support Vector Machine trained on phasor coordinates) integrates the “D-trajectory” (developmental trajectory) to assign a scalar Viability Score (0-100) to each embryo.
Economic Impact Analysis for ART Centers
Adopting METAPHOR represents a capital expenditure but offers a compelling Return on Investment (ROI) through three primary channels: increased success rates, reduced operational costs, and premium service differentiation.
1. The “Failed Cycle” Cost Reduction
The true cost of IVF is not the cycle itself, but the cumulative cost of failed transfers.
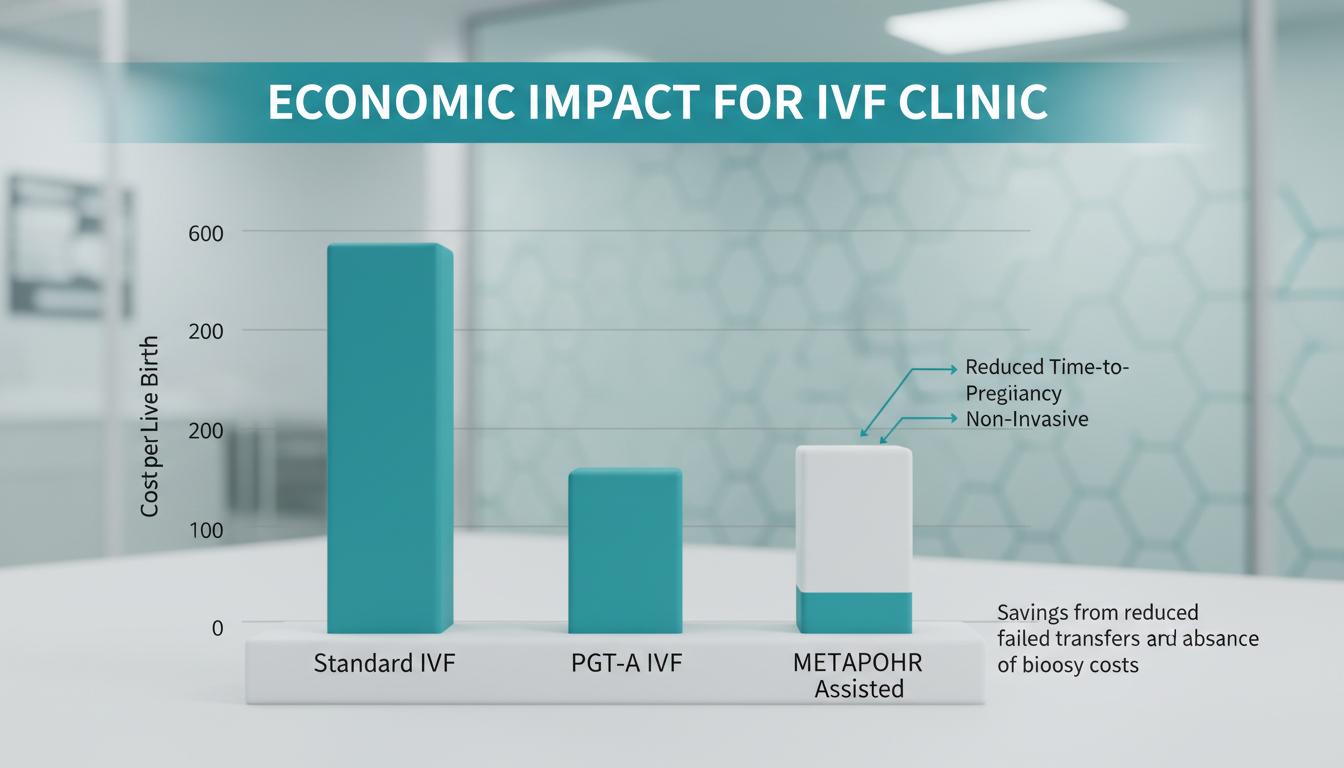
- Current State: A patient may undergo 3 transfers to achieve a live birth. Each failed frozen embryo transfer (FET) costs the clinic overhead and the patient ~$4,000.
- METAPHOR Impact: By improving the ranking of embryos, the system reduces the average number of transfers to live birth (TTL) from ~2.5 to ~1.4.
- Savings: For a clinic performing 1,000 cycles/year, avoiding just one failed transfer per patient represents an efficiency gain of over $1.5M in liberated clinic capacity and reduced medication costs.
2. Displacing Invasive PGT-A
While PGT-A generates revenue, it also consumes significant embryologist time (biopsy, tubing) and incurs external lab fees ($200-$300 per embryo).
- Cost-Benefit: A non-invasive metabolic scan takes minutes and costs <$50 in consumables (media/dish).
- Patient Access: By lowering the price point of “advanced selection” from ~$5,000 (PGT-A) to ~$800 (Metabolic Scan), clinics can capture a massive segment of the market (e.g., younger patients, <35) who currently opt out of PGT-A but desire higher assurance than morphology.
3. Workflow Efficiency
The “phasor” analysis is computationally instantaneous. Unlike time-lapse systems that require 5 days of continuous monitoring and massive data storage, METAPHOR can be used as a “snapshot” diagnostic on Day 5, integrating seamlessly into the existing workflow prior to vitrification or transfer.
Future Regulatory Path: From Research to Standard of Care
Classification and Predicate
METAPHOR falls under the Class II Medical Device category (FDA) as a Computer-Aided Triage and Notification (CADt) or Clinical Decision Support (CDS) software, coupled with a hardware optical system.
- Predicate Devices: It will likely reference predicates such as the Eeva Test (early time-lapse AI) or other FLIM-based ophthalmic devices, though the De Novo pathway is probable given the novel use of hyperspectral metabolic imaging in IVF.
Software as a Medical Device (SaMD) Considerations
The FDA’s recent “Predetermined Change Control Plan” (PCCP) guidance is crucial here. METAPHOR’s AI models must be “locked” for initial approval, but the manufacturer must submit a PCCP detailing how the model will be retrained and improved with real-world data without requiring a new 510(k) for every iteration.
Validation Hurdles
To achieve widespread adoption, multi-centric Randomized Controlled Trials (RCTs) must demonstrate non-inferiority to PGT-A in selecting euploid embryos and superiority to morphology in Live Birth Rate (LBR). The safety profile of 780nm two-photon exposure is well-established in animal models, but long-term epigenetic safety data in humans will be the final hurdle for full regulatory clearance.

Conclusion: The Metabolic Era of IVF
Phasor-based hyperspectral metabolic imaging represents the transition of embryology from a descriptive art to a quantitative science. By looking beyond the physical structure of the embryo and into its molecular engine, METAPHOR addresses the fundamental inefficiency of IVF: the inability to see life before it begins.
For the biomedical engineer, it is a triumph of photonics and signal processing. For the clinic owner, it is a strategic asset that reduces risk and maximizes throughput. But for the patient, it offers the most valuable commodity of all: a shorter, clearer path to parenthood. As the technology matures from the bench to the incubator, “metabolic competence” will undoubtedly supersede morphology as the primary language of embryo selection.
