Executive Summary
In the rapidly evolving landscape of Assisted Reproductive Technology (ART), the standardization of “Day 0” procedures—specifically oocyte denudation—remains a critical yet undervalued frontier. While enzymatic digestion and manual pipetting have served as the clinical standard for decades, they introduce significant operator-dependent variability and potential mechanical stress to the oocyte. This clinical deep-dive explores the emergence of Vibration-Induced Flow (VIF) automation, a paradigm shift utilizing Piezoelectric Micro-Bioreactors (PMBs) to achieve precise, non-invasive cumulus cell removal.
Emerging data from late 2024 and 2025 indicates that VIF-based automated denudation not only matches manual efficacy in fertilization and blastocyst formation rates but significantly reduces shear stress exposure by approximately ten-fold. By leveraging piezoelectric actuation to generate controlled “whirling flows” around micropillar arrays, these devices mechanically dissociate the cumulus-oocyte complex (COC) while preserving the delicate meiotic spindle and zona pellucida. For ART clinics, this technology represents a dual opportunity: the biological optimization of gamete handling and the economic rationalization of laboratory workflows through automation.
detailed Problem Statement: The Manual Denudation Bottleneck
The Clinical Perspective: Variability and Toxicity
From a clinical embryology standpoint, oocyte denudation is a high-stakes procedure. It is the prerequisite for Intracytoplasmic Sperm Injection (ICSI), the dominant fertilization method globally. The goal is to remove the cumulus-corona radiata mass to assess nuclear maturity (Metaphase II status) and enable precise micropipette access.
However, the current “gold standard” is fraught with inconsistencies:
- Enzymatic Toxicity: The procedure typically relies on hyaluronidase to digest the extracellular matrix. Prolonged exposure—often unavoidable when processing large cohorts—can lead to oocyte activation or cytoplasmic toxicity.
- Mechanical Stress: Following enzymatic exposure, embryologists perform mechanical stripping using narrow-bore pipettes. The shear stress exerted during this aspiration-expulsion cycle is highly operator-dependent. Inexperienced hands can apply peak shear forces exceeding 40–50 Pascals (Pa), potentially damaging the oolemma or disrupting the transzonal projections (TZPs) too aggressively.
- Subjectivity: There is no standardized metric for “complete” denudation. Residual cumulus cells can interfere with the holding pipette seal during ICSI, while over-stripping can compromise the oocyte’s intrinsic developmental potential.
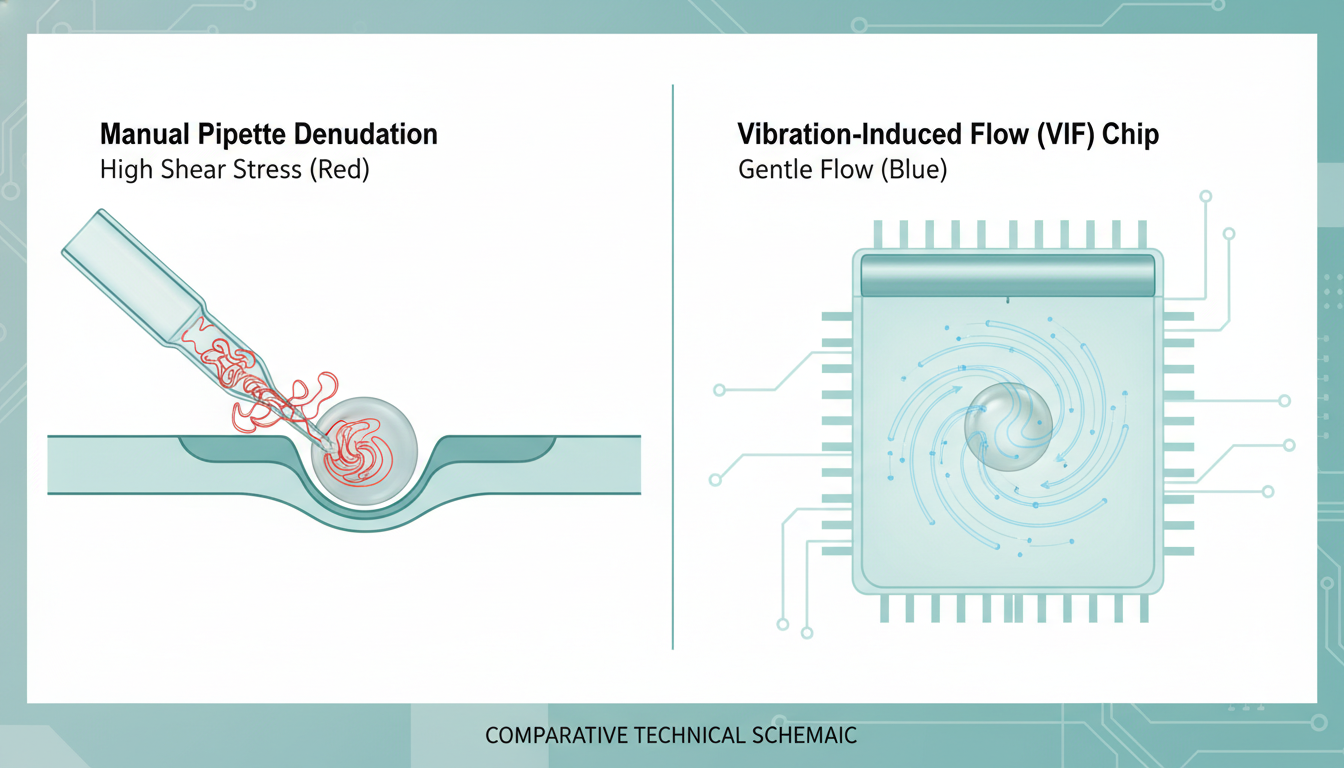
The Engineering Perspective: Fluid Dynamics and Control
From a biomedical engineering perspective, manual pipetting is a chaotic fluid dynamic event. The flow profile within a stripping pipette is parabolic but pulsatile, driven by manual pressure. The oocyte, effectively a large viscoelastic particle, is subjected to rapid deformation and tumbling. The lack of real-time feedback means the shear stress profile is unknown and uncontrolled.
The challenge, therefore, is to decouple the removal force (needed to break intercellular junctions) from the transport force (which moves the oocyte). Manual pipetting couples them; if you suck harder to move the egg, you strip harder. Piezoelectric automation seeks to separate these vectors.
Theoretical Framework & Molecular Mechanism
The Piezoelectric Actuation Principle
At the core of the VIF solution is the Piezoelectric Micro-Bioreactor (PMB). Unlike traditional microfluidics that rely on syringe pumps (pressure-driven flow), PMBs utilize piezoelectric transducers—often Lithium Niobate (LiNbO3) substrates—to generate acoustic waves or mechanical vibrations.
In the VIF configuration, the device typically features an open-surface or enclosed chamber containing an array of micropillars. When the piezoelectric actuator vibrates the substrate at a specific resonant frequency (often in the low kHz to MHz range), it induces a secondary fluid flow around these pillars. This is known as acoustic streaming or vibration-induced whirling flow.
Key Hydrodynamic advantages:
1. Local Flow Generation: The fluid motion is generated locally around the pillars, not by a global pressure gradient. This means the oocyte does not need to be pumped through long, narrow channels where clogging or crushing can occur.
2. Shear Stress Modulation: The “whirling” vortices created by the pillars exert a constant, low-magnitude shear stress on the COC. Recent computational fluid dynamics (CFD) simulations suggest this automated shear stress peaks at approximately 4.4 Pa—roughly 10% of the stress exerted during vigorous manual pipetting.
Molecular Disassociation Dynamics
To understand why this gentle vibration works, we must look at the molecular architecture of the COC. The cumulus mass is anchored to the oocyte via two primary mechanisms:
1. The Hyaluronic Acid (HA) Matrix: A viscoelastic scaffold stabilized by proteins such as pentraxin-3 (PTX3), TSG-6, and inter-alpha-inhibitor. Hyaluronidase softens this matrix, but mechanical force is required to strip the “sticky” remnants.
2. Gap Junctions (Connexin-43): These are the intercellular channels connecting the cumulus cells to the oocyte via Transzonal Projections (TZPs) through the zona pellucida. These junctions are the “rivets” holding the inner corona radiata layer.
Under VIF automation, the oscillatory nature of the fluid flow fatigues these molecular bonds. Instead of a single high-force “rip” (as in pipetting), the VIF applies thousands of micro-tugs per second. This fatigue-based delamination allows for the disruption of Connexin-43 gap junctions and the HA matrix without exceeding the yield strength of the oocyte’s zona pellucida or plasma membrane.

Review of Device Technology & Engineering
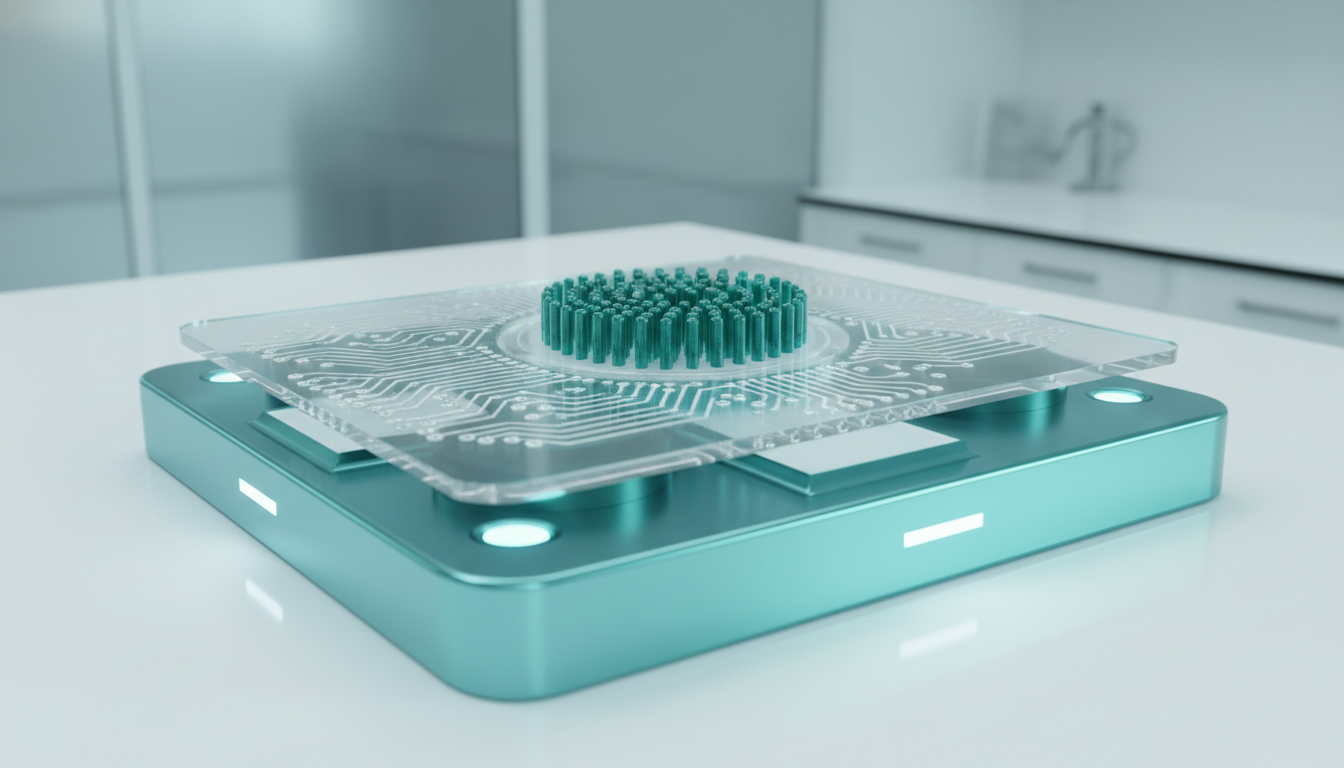
Device Architecture: The “Chip” Design
The prevailing design for VIF denudation chips, as highlighted in recent 2025 literature, centers on a disposable microfluidic cartridge.
- The Loading Chamber: A central reservoir where the COCs are deposited. This chamber is often open-access to allow standard embryologist workflow (pipette loading).
- The Pillar Array: A spiral or concentric arrangement of SU-8 or PDMS (polydimethylsiloxane) micropillars. The geometry is critical; the pillar spacing (gap size) is tuned to be smaller than the oocyte (~120 µm) but larger than cumulus cells (~10-20 µm).
- The Vibration Source: The chip docks into a reusable base station housing the piezoelectric actuator. The base station controls the frequency (Hz) and amplitude (Voltage) of the vibration.
Operational Workflow
1. Incubation: COCs are briefly exposed to a hyaluronidase solution to soften the ECM.
2. Loading: The COCs are transferred to the VIF chip reservoir.
3. Actuation: The piezo element is activated for a preset duration (e.g., 10–30 seconds). The vibration induces the whirling flow. The heavy, large oocytes are retained within the center or specific “traps” due to their inertia and size, while the smaller, lighter cumulus cells are swept away by the drag forces of the streaming flow.
4. Collection: The clean oocytes are aspirated from the collection well, ready for ICSI.
Comparative Performance Data
Recent studies (e.g., Cornell, Lab on a Chip 2025) have validated this approach against manual controls. Key performance indicators include:
- Fertilization Rate: 93.1% (VIF) vs. 90.7% (Manual). No statistically significant difference, indicating safety.
- Blastocyst Formation: 43.1% (VIF) vs. 50.0% (Manual). Comparable development potential.
- Throughput: The ability to process cohorts of up to 23 oocytes simultaneously, contrasting with the serial “one-by-one” nature of manual stripping.
Economic Impact Analysis for ART Centers
The adoption of Piezoelectric Micro-Bioreactors is not merely a clinical upgrade; it is a strategic business decision. The economic argument rests on three pillars: Labor Efficiency, Standardization, and Risk Mitigation.
1. Labor Efficiency and Throughput
In a high-volume IVF center performing 1,000+ cycles annually, oocyte denudation represents a significant chunk of senior embryologist time. Manual denudation of a cohort of 10–15 eggs can take 15–20 minutes of intense concentration.
- VIF Efficiency: Automated chips can process the entire cohort in <5 minutes (including loading/unloading).
- FTE Savings: This frees up approximately 15 minutes per case. Over 1,000 cases, this aggregates to ~250 hours of senior embryologist time annually—equivalent to roughly $25,000–$35,000 in direct labor costs, or the capacity to perform an additional 50–60 ICSI procedures.
2. Training and Standardization
Training a junior embryologist to perform manual denudation without damaging eggs takes months of supervision. The “learning curve” often involves subtle damage to donor eggs or practice material.
- De-skilling the Step: VIF automation turns a high-skill manual art into a push-button protocol. A junior technician can load the chip, run the cycle, and retrieve oocytes with the same consistency as a lab director.
- Outcome Consistency: By removing the “human hand” variable, clinics can standardize their denudation results across different staff members, reducing the Coefficient of Variation (CV) in fertilization rates between operators.
3. Risk Mitigation
One of the silent costs in IVF is the “lost oocyte” or “damaged oocyte” during handling. If a rough denudation leads to oocyte degeneration (atresia) in 5% of cases, that directly impacts the cumulative pregnancy rate.
- Liability Reduction: Automating the process provides a digital log of the procedure (time, vibration intensity), offering quality control traceability that manual pipetting cannot provide.
Future Regulatory Path
FDA Classification and Predicate Devices
For the US market, VIF denudation devices will likely fall under Class II medical devices.
- Product Code: Likely MQK (Labware, Microdissection, Assisted Reproduction) or a new code specific to “Automated Cell Separation for ART.”
- 510(k) Strategy: The regulatory pathway will involve demonstrating Substantial Equivalence (SE) to existing manual denudation pipettes (e.g., The Stripper®) and perhaps prior microfluidic sperm sorting devices (like the ZyMōt).
Safety and Efficacy Requirements
To secure clearance, manufacturers must generate data proving:
1. MEA (Mouse Embryo Assay): Showing that the device material (PDMS, plastic) and the piezoelectric energy do not impair blastocyst development (pass criteria usually >80%).
2. LAL (Endotoxin): Standard biocompatibility.
3. Shear Stress Validation: CFD modeling and real-world validation proving the forces are within the physiological safety window (<10 Pa).
4. Genetic Safety: Ideally, verifying that the vibration does not induce aneuploidy (spindle disruption), although healthy live birth data in mouse models is usually the primary threshold for initial clearance.

Conclusion
Vibration-Induced Flow automation via Piezoelectric Micro-Bioreactors represents a maturing frontier in the “Lab of the Future.” It addresses the fundamental paradox of modern IVF: we use advanced lasers and AI for embryo selection, yet we rely on 1980s-era mouth-pipetting for the delicate initial step of oocyte processing.
By leveraging the physics of acoustic streaming and fatigue-based molecular detachment, VIF technology offers a gentle, scalable, and reproducible alternative to manual denudation. For the ART clinic, the value proposition is clear: improved biological safety for the gamete, reduced operational variance for the lab, and a tangible step toward the fully automated, standardized fertility center. As these devices move from academic prototypes to commercial products in late 2025 and 2026, they will likely become a standard fixture in the high-performance embryology workstation.
