Executive Summary
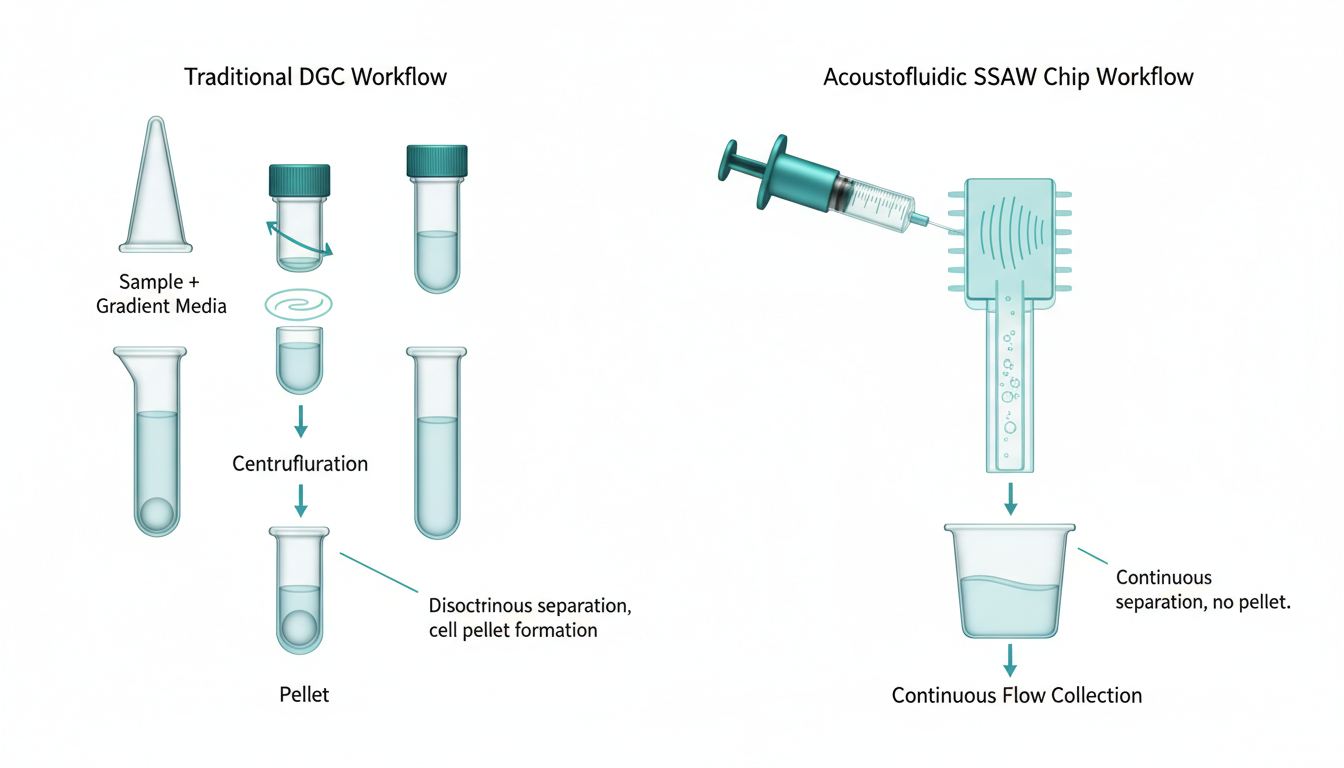
The landscape of Assisted Reproductive Technology (ART) is currently undergoing a paradigm shift, moving away from centrifugation-based sperm preparation toward microfluidic and acoustofluidic isolation. While traditional Density Gradient Centrifugation (DGC) and ‘Swim-Up’ techniques have served as the gold standard for three decades, emerging data from 2024 and 2025 indicates they may inadvertently contribute to iatrogenic DNA damage via Reactive Oxygen Species (ROS) generation.
This deep-dive analysis focuses on Standing-Surface Acoustic Waves (SSAW)—colloquially termed “Acoustic Sperm Tweezing”—as a non-invasive, label-free, and high-throughput alternative. Unlike optical tweezers, which risk thermal damage to the gamete, acoustic tweezers utilize low-power ultrasonic fields to manipulate cells based on compressibility, density, and size.
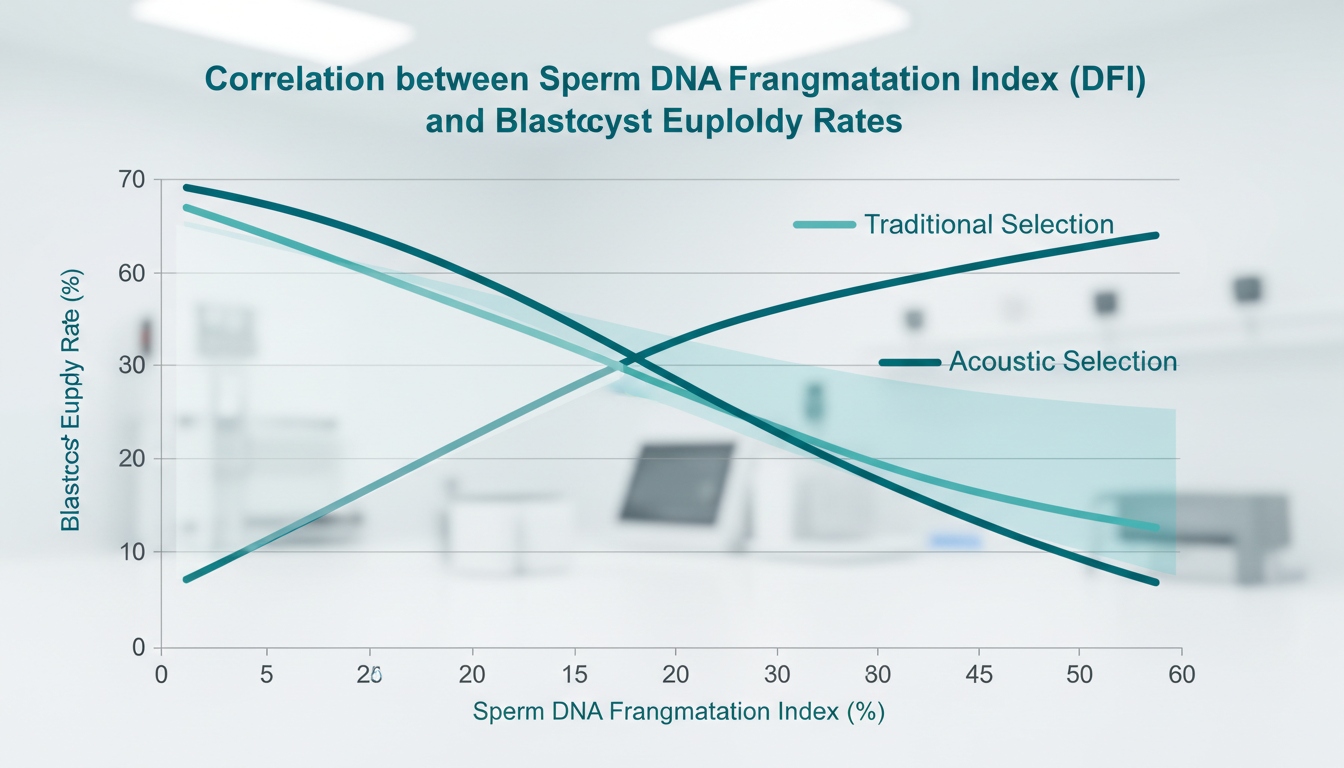
Recent clinical validations suggest that SSAW devices can isolate spermatozoa with significantly lower DNA Fragmentation Indices (DFI) (<5%) compared to DGC (>15-20%), directly correlating with improved blastocyst euploidy rates. For the modern IVF clinic, the adoption of acoustofluidics represents not just a technological upgrade, but a fundamental change in how male factor infertility is managed—shifting from “washing” sperm to “biophysically selecting” the optimal gamete for Intra-Cytoplasmic Sperm Injection (ICSI).
Detailed Problem Statement: The ‘Hidden’ Male Factor
The Clinical Perspective: Beyond the Spermiogram
For decades, the World Health Organization (WHO) guidelines for semen analysis have dictated the clinical approach to male infertility. Clinicians focus on concentration, motility, and morphology. However, a growing body of literature identifies a disconnect: a patient may present as “Normozoospermic” yet repeatedly fail IVF cycles or suffer recurrent pregnancy loss (RPL). The culprit is often Sperm DNA Fragmentation (SDF), a molecular defect invisible to standard light microscopy.
Clinics currently rely on DGC to prepare samples. While DGC effectively concentrates sperm, it subjects the cells to high G-forces (typically 300-400g) and pelleting. This mechanical stress, combined with the removal of seminal plasma antioxidants, creates an oxidative environment promoting ROS production. In effect, the very process used to prepare sperm may be compromising its genomic integrity.
The Laboratory Perspective: Workflow & Standardization
From an embryology lab standpoint, sperm preparation is labor-intensive and highly operator-dependent. The “Swim-Up” method, while gentler than DGC, has low yield and is impractical for severe oligozoospermic samples. Furthermore, these manual methods lack standardization; the quality of the pellet varies between technicians.
There is an urgent unmet need for a device that is:
1. Automated: reducing operator variability.
2. High-Yield: capable of recovering sufficient sperm for ICSI from sub-optimal samples.
3. Genomically Protective: avoiding centrifugation and oxidative stress.
Theoretical Framework: The Physics of Acoustic Tweezing
Governing Equations of Acoustophoresis
The fundamental mechanism behind SSAW sperm selection is acoustophoresis, the migration of particles using acoustic radiation force (ARF). When a standing wave is generated inside a microfluidic channel, it creates pressure nodes (minima) and antinodes (maxima).
The primary acoustic radiation force ($F_{rad}$) acting on a spherical particle (the sperm head) in a standing wave field is given by:
$$ F_{rad} = – \left( \frac{\pi p_0^2 V_c \beta_f}{2\lambda} \right) \Phi(\beta, \rho) \sin(2kx) $$
Where:
- $p_0$ is the acoustic pressure amplitude.
- $V_c$ is the volume of the cell (sperm head).
- $\beta_f$ is the compressibility of the fluid medium.
- $\lambda$ is the acoustic wavelength.
- $k$ is the wavenumber ($2\pi/\lambda$).
- $\Phi$ is the acoustic contrast factor, defined by the density ($\rho$) and compressibility ($\beta$) differences between the cell and the medium.
The Acoustic Contrast Factor ($\Phi$)
The sign of $\Phi$ determines the direction of migration.
- If $\Phi > 0$, particles move toward pressure nodes.
- If $\Phi < 0$, particles move toward pressure antinodes.
Spermatozoa generally have a positive contrast factor relative to standard IVF media, meaning they migrate toward the pressure nodes. However, the magnitude of the force depends on the cell volume ($V_c$). This is the critical “tweezing” mechanism: morphologically normal sperm (with specific head dimensions and density) experience a different magnitude of force compared to debris, white blood cells, or morphologically abnormal sperm.
Competition of Forces: Motility vs. Acoustics
In a static particle scenario, all cells would simply aggregate at the nodes. However, sperm are active swimmers. In an SSAW device, the design purposely creates a competition between the Acoustic Radiation Force and the sperm’s intrinsic Motility (Hydrodynamic Drag).
- Dead/Immotile Sperm: Dominated by the acoustic force and Stokes’ drag from the fluid flow. They are pushed strictly to the pressure nodes (often the channel walls or center, depending on design).
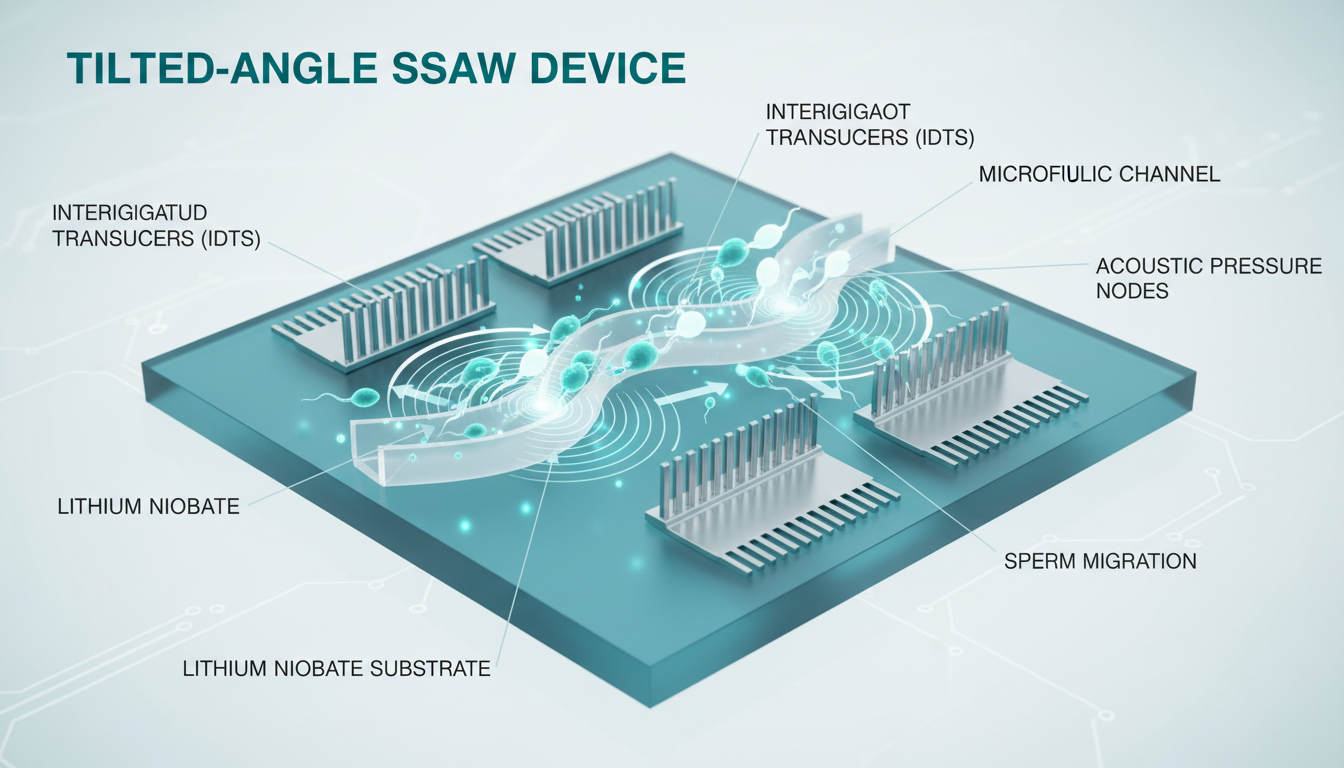
- High-Motility Sperm: Possess sufficient flagellar propulsion to “break” the acoustic trap or swim across the streamlines. By tilting the acoustic field relative to the flow direction (Tilted-Angle SSAW), engineers can force motile sperm to swim against or across the acoustic gradient, effectively filtering them based on swimming strength—a proxy for mitochondrial function and genomic health.
Device Technology & Engineering Architecture
Substrate and Transducers
The core of an SSAW sperm sorter is a piezoelectric substrate, typically Lithium Niobate ($LiNbO_3$). This material couples electrical energy into mechanical vibration with high efficiency.
Interdigitated Transducers (IDTs) are metallic electrodes (usually Gold or Titanium) photolithographically patterned onto the substrate. When an RF signal (Radio Frequency, typically 10-40 MHz) is applied to the IDTs, they generate Surface Acoustic Waves (SAW) that propagate along the substrate surface. Two opposing IDTs generate identical waves traveling toward each other, interfering to form a Standing Surface Acoustic Wave (SSAW).
Microfluidic Channel Design
A Polydimethylsiloxane (PDMS) microchannel is bonded on top of the substrate. The channel geometry is critical:
1. Sheath Flow: A central sample inlet is flanked by two buffer inlets. This focuses the sperm sample into a narrow stream before it enters the acoustic field.
2. Tilted Angle Geometry: The IDTs are often angled (e.g., 15° to 30°) relative to the fluid flow. This setup ensures that as cells flow through the channel, they experience the acoustic force vector laterally.
- Result: Large, immotile cells (leukocytes, debris) are laterally displaced the most (following the acoustic nodes).
- Result: Highly motile sperm fight this displacement, maintaining a different trajectory that leads them to a separate “Selected” outlet.
Thermal Management
One engineering challenge is heat. Piezoelectric actuation generates heat, which is detrimental to sperm (sperm are highly thermosensitive). Advanced SSAW devices employ:
- Pulsed Actuation: Cycling the RF signal (e.g., 50ms ON, 50ms OFF) to dissipate heat while maintaining time-averaged acoustic forces.
- Active Cooling: Peltier elements or heatsinks integrated into the device holder to maintain the medium at exactly 37°C (physiological) or slightly lower (RT) to reduce metabolic stress during sorting.
Molecular Mechanism: Why SSAW Preserves DNA
The ‘Soft’ Physical Trap
Unlike centrifugation, which subjects the sperm to mechanical crushing forces (pelleting), acoustofluidics exerts a gentle, distributed pressure field. The acoustic pressure amplitude used in these devices (typically <1 MPa) generates forces in the piconewton (pN) range. This is sufficient to guide the cell but insufficient to cause shear stress damage to the plasma membrane or the acrosome cap.
Exclusion of Reactive Oxygen Species (ROS)
In a standard centrifuge pellet, leukocytes and dead sperm are forced into close proximity with viable sperm. Leukocytes are potent generators of ROS (superoxide anions, hydrogen peroxide). Even a short duration in a pellet can cause oxidative attacks on the sperm DNA backbone, leading to single- and double-strand breaks.
In SSAW sorting:
1. Continuous Flow: There is no pelleting. Sperm are constantly moving in a laminar flow stream.
2. Spatial Separation: Leukocytes are acoustically separated from the viable sperm almost instantly (within milliseconds of entering the field).
3. Result: The “Selected” sperm fraction has never been in prolonged contact with the high-ROS “Waste” fraction.
Recent comparative studies (2023-2025) utilizing the TUNEL assay and Sperm Chromatin Structure Assay (SCSA) have shown that SSAW-selected sperm exhibit DFI levels comparable to raw neat semen (baseline) or even improved, whereas DGC-processed sperm often show elevated DFI due to the processing itself.
Economic Impact Analysis for ART Centers
Transitioning from disposable columns to an acoustofluidic platform involves capital expenditure (CapEx) but offers significant operational expenditure (OpEx) benefits and ROI based on clinical outcomes.
1. Throughput and Labor Efficiency
- Current State: A standard swim-up or gradient takes 45-90 minutes of technician time (layering, centrifuging, washing, incubating).
- SSAW State: Automated processing. The technician loads the sample and presses “Start.” The device runs for 20-40 minutes (depending on volume) unattended.
- Impact: An embryologist can perform ICSI or vitrification while the sperm is being sorted. This parallelization saves approximately 1 hour of highly skilled labor per cycle.
2. Disposable Costs vs. CapEx
- Traditional: Gradients and wash media cost ~$50-$80 per cycle.
- Acoustofluidic: The microfluidic chip is the consumable. Current market pricing for similar microfluidic devices (e.g., membrane-based) is ~$150-$200.
- Analysis: The consumable cost is higher. However, the reduction in media use (no large volume washing) partially offsets this.
3. The ‘Success Rate’ Multiplier
The true economic driver is the Live Birth Rate (LBR).
- If SSAW sorting reduces the miscarriage rate by selecting DNA-intact sperm, the clinic improves its “Baby-Take-Home” statistics.
- Metric: A 5% increase in LBR allows a clinic to command premium pricing or attract higher volume.
- Reduction in Failed Cycles: For a patient, a failed IVF cycle costs ~$15,000-$20,000. If better sperm selection prevents one failed cycle, the cost-benefit for the patient is overwhelming. Clinics can market “Premium Sperm Selection” as an add-on service (e.g., $500 surcharge), which patients are willing to pay to mitigate the risk of cycle failure.
Future Regulatory Path & Clinical Implementation
FDA and CE Mark Landscape
As of late 2025, most microfluidic sperm sorters fall under Class II medical devices in the US (FDA). The predicate devices are typically simple filtration or membrane-based systems (like the ZyMōt).
SSAW Specific Challenges:
- Active Energy: Unlike passive membrane chips, SSAW devices use active ultrasonic energy. The FDA requires stringent safety data regarding thermal effects and potential cavitation (bubble formation that can damage cells).
- Biocompatibility: The piezoelectric material ($LiNbO_3$) is generally not in direct contact with the fluid (separated by a coupling layer or the microchannel floor), but the bonding agents and PDMS must be USP Class VI certified.
The Path to ‘Standard of Care’
For SSAW to replace DGC, large-scale Randomized Controlled Trials (RCTs) are needed. Current studies are often single-center cohorts. The key endpoints for regulatory and clinical acceptance are:
1. Non-inferiority in Fertilization Rate: Proving SSAW yields enough sperm for ICSI (easy) or conventional IVF (harder due to volume requirements).
2. Superiority in Euploidy: Demonstrating that SSAW cohorts have higher PGT-A normal blastocyst rates.
Integration into the ‘Lab on a Chip’ Ecosystem
The future vision is the “Integrated IVF Chip.” Imagine a device where:
1. Raw semen is input at Port A.
2. SSAW sorts the sperm.
3. Selected sperm flow directly into a droplet generation module where they are encapsulated with a single oocyte (microfluidic IVF).
4. No manual pipetting occurs until the embryo stage.
This level of automation would revolutionize the scalability of IVF, making it accessible to a broader demographic by reducing the manual labor overhead.
Conclusion
Acoustofluidic ‘Sperm Tweezing’ via Standing-Surface Acoustic Waves represents the convergence of precision physics and reproductive medicine. By abandoning the brute force of the centrifuge in favor of the gentle precision of the acoustic node, we align clinical practice with biological imperative: preserving the genomic integrity of the male gamete.
For the infertility specialist, the message is clear: the era of “washing” sperm is ending. The era of “selecting” sperm based on biophysical competence—without chemical markers or mechanical trauma—has arrived. While the engineering is complex, the clinical benefit is simple: healthier sperm, better embryos, and ultimately, higher success rates for patients struggling to conceive.

