Executive Summary
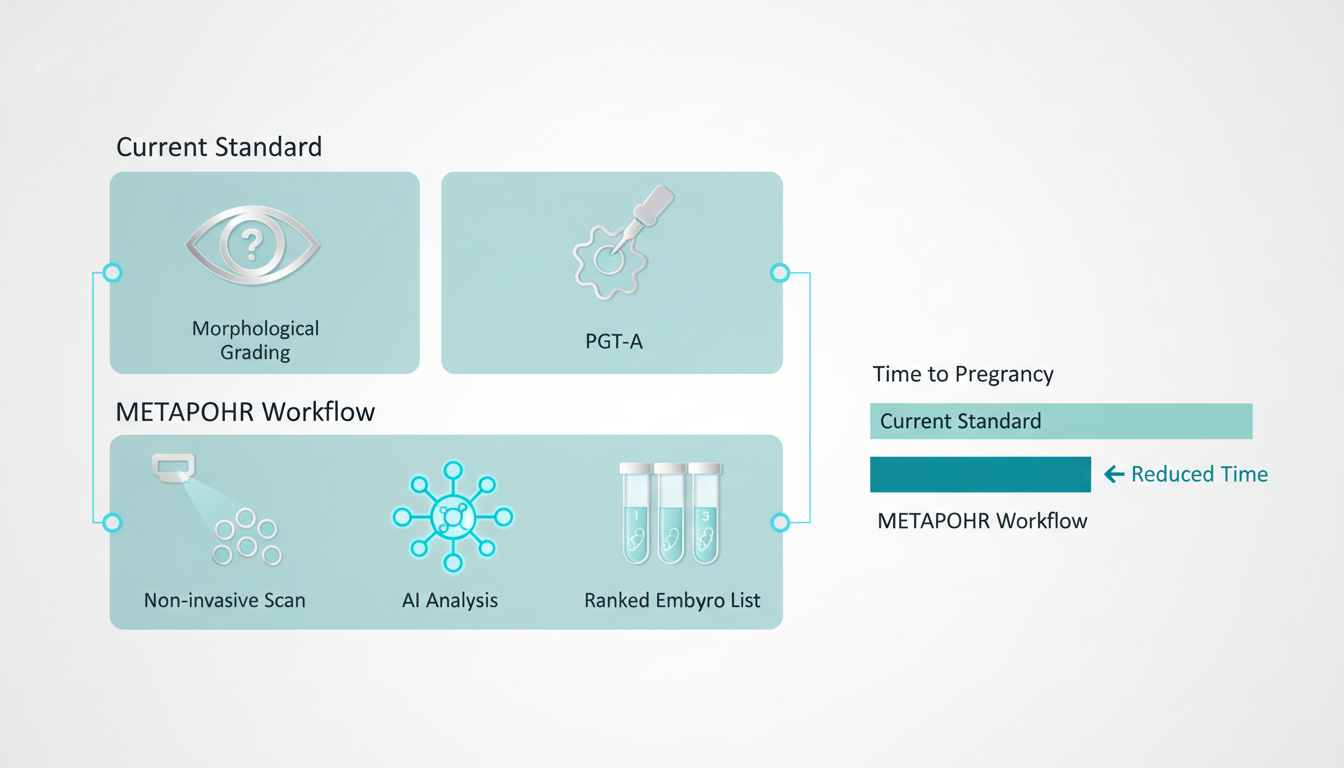
In the landscape of Assisted Reproductive Technology (ART), the selection of the most viable embryo remains the single most critical determinant of clinical pregnancy and live birth rates. Despite advancements in time-lapse morphokinetics and preimplantation genetic testing for aneuploidy (PGT-A), the field lacks a truly non-invasive, functional assay of embryo physiology. Current selection methods rely either on subjective morphological grading—which correlates poorly with metabolic health—or invasive biopsies that impose mechanical stress on the developing blastocyst.
METAPHOR (Metabolic Evaluation Through Phasor-based Hyperspectral Organelle Recognition) represents a paradigm shift in embryology. By combining two-photon hyperspectral imaging with artificial intelligence (AI), METAPHOR provides a direct, quantitative read-out of the embryo’s metabolic state without the need for exogenous labels or contrast agents. This technology leverages the intrinsic autofluorescence of metabolic coenzymes—specifically Nicotinamide Adenine Dinucleotide (NAD(P)H) and Flavin Adenine Dinucleotide (FAD)—to map the spatial distribution of oxidative phosphorylation versus glycolysis within individual blastomeres.
Early validation studies, including pivotal data published in PNAS (2024), demonstrate that METAPHOR can discriminate between oocytes of varying maternal ages with 96% accuracy and predict blastocyst formation with over 80% accuracy—metrics that significantly outperform standard morphological evaluation. This deep-dive analysis explores the biophysical mechanisms, engineering architecture, and clinical economic implications of integrating METAPHOR into the modern IVF laboratory.
Detailed Problem Statement: The “Black Box” of Embryo Metabolism
The Clinical Perspective: Subjectivity and Invasiveness
For the Reproductive Endocrinologist (REI), the current standard of care presents a dichotomy of risk and uncertainty.
1. Morphological Grading (Gardner Scale): While non-invasive, this method is inherently subjective. An embryo graded “4AA” by one embryologist may be deemed “4AB” by another. Crucially, morphology is a lagging indicator; an embryo may appear structurally sound while suffering from profound metabolic energetic failure that precludes implantation.
2. PGT-A (Biopsy): While offering genetic clarity regarding euploidy, PGT-A requires the removal of trophectoderm cells. This procedure adds significant cost ($3,000–$5,000 per cycle), requires highly skilled practitioners, and carries a non-zero risk of damaging the embryo. Furthermore, PGT-A does not assess metabolic viability; a euploid embryo can still fail to implant due to mitochondrial dysfunction.
The Laboratory Perspective: The Metabolic Switch
From an embryologist’s standpoint, the preimplantation embryo undergoes a critical metabolic transition.
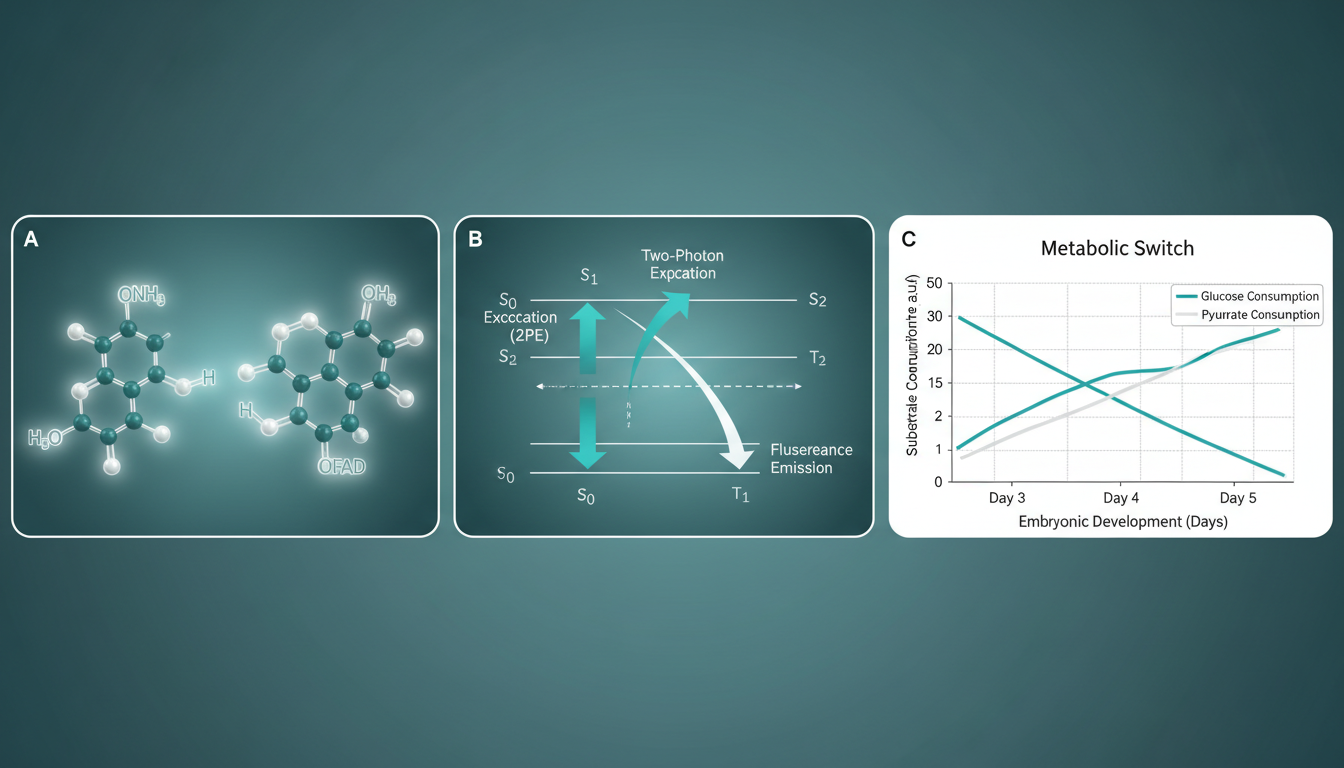
- Cleavage Stage (Day 1-3): The embryo relies primarily on pyruvate and lactate via oxidative phosphorylation.
- Blastocyst Stage (Day 4-5): As the embryo differentiates into the Inner Cell Mass (ICM) and Trophectoderm (TE), there is a sharp increase in glucose uptake and a shift toward aerobic glycolysis (the Warburg effect) to support rapid proliferation.
Failure to execute this “metabolic switch” is a hallmark of developmental incompetence. Current laboratory tools (brightfield microscopy, time-lapse) are blind to these molecular events. The embryo remains a metabolic “black box”—its energetic potential inferred only from the rate of cell division. There is an urgent unmet need for a tool that can visualize this metabolic machinery in real-time, non-invasively, and in 3D.

Theoretical Framework & Molecular Mechanism
METAPHOR operates at the intersection of biophotonics and biochemistry, utilizing the phenomenon of autofluorescence to generate metabolic contrast.
1. The Fluorophores: NAD(P)H and FAD
Cellular metabolism relies on redox reactions catalyzed by two primary coenzymes:
- NAD(P)H (Reduced Nicotinamide Adenine Dinucleotide): Fluoresces when excited by UV/blue light (absorption ~340nm, emission ~460nm). It is the primary electron donor in the electron transport chain (ETC).
- FAD (Oxidized Flavin Adenine Dinucleotide): Fluoresces in the green-yellow range (absorption ~450nm, emission ~535nm). It acts as an electron acceptor.
Crucially, the fluorescence quantum yield and spectral signature of these molecules change depending on their binding state (protein-bound vs. free). This allows for the differentiation of metabolic pathways.
2. The Optical Redox Ratio
The Optical Redox Ratio (ORR), typically defined as $FAD / (NAD(P)H + FAD)$, serves as a quantitative biomarker of cellular metabolism.
- A low redox ratio typically indicates high levels of NADH, associated with glycolysis and proliferation (typical of healthy blastocysts).
- A high redox ratio suggests reliance on oxidative phosphorylation or oxidative stress.
However, intensity-based ratios alone are susceptible to artifacts from signal attenuation and concentration differences. This is where METAPHOR’s Phasor-based analysis becomes revolutionary.
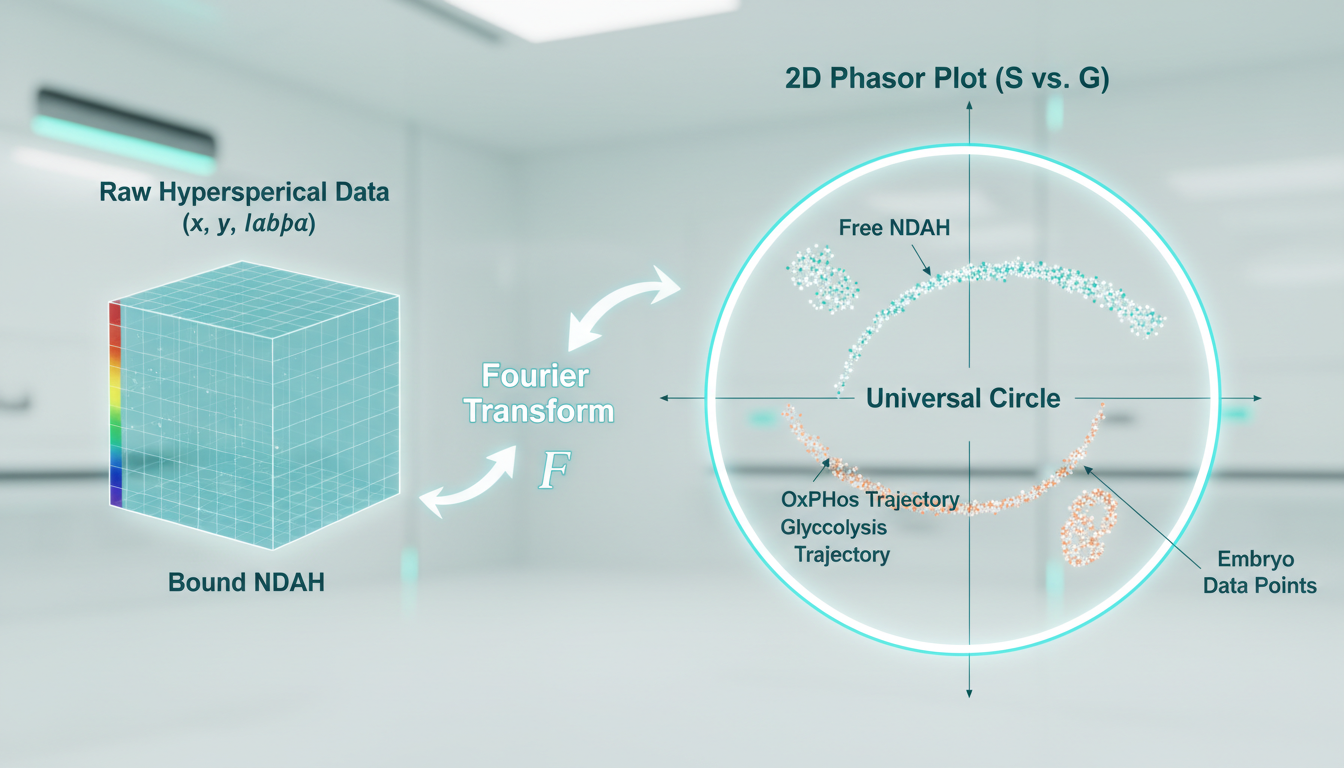
3. Spectral Phasor Analysis
Unlike traditional hyperspectral unmixing, which requires prior knowledge of end-member spectra, Phasor Analysis transforms the spectral data from every pixel into a vector on a 2D plot (the phasor plot).
- The Math: The spectral emission $I(\lambda)$ at each pixel is Fourier transformed to calculate coordinates $G$ (real axis) and $S$ (imaginary axis).
- The Plot: Pixels with identical spectral shapes cluster together in the phasor plot, regardless of intensity.
- Metabolic Trajectory: Pure free NADH and pure protein-bound NADH fall at specific points on the “universal circle.” A pixel containing a mixture of both will fall on the line connecting them. By analyzing the position of embryo pixels on this trajectory, METAPHOR can quantify the exact fraction of bound vs. free NAD(P)H, offering a precise readout of the balance between glycolysis and oxidative phosphorylation.
Review of Device Technology & Engineering
The METAPHOR system described in recent high-impact literature (e.g., HYLIGHT project reports, PNAS) is not merely a camera but a sophisticated opto-electronic platform.
1. Excitation Source: Two-Photon Microscopy (2PE)
To ensure safety and depth penetration, METAPHOR utilizes Two-Photon Excitation.
- Wavelength: Typically ~740nm–780nm (Near-Infrared).
- Safety: Unlike UV light (which excites NAD(P)H but damages DNA), NIR light is non-ionizing. Two-photon absorption occurs only at the focal point, minimizing phototoxicity to the surrounding blastomeres. This is critical for clinical adoption, as the safety margin for human embryos is non-negotiable.
2. Hyperspectral Detection
Instead of standard bandpass filters (e.g., “Blue” vs “Green” channels), the system employs a hyperspectral detector (often a specialized spectral camera or a multi-anode PMT with dispersive optics).
- Data Cube: The system captures a $(x, y, \lambda)$ data cube, where every spatial pixel contains a full emission spectrum (e.g., 400nm to 650nm in 10nm steps).
- Resolution: High spatial resolution is required to segment individual organelles (mitochondria) within the blastomeres.
3. AI-Driven Organelle Recognition
The “OR” in METAPHOR stands for Organelle Recognition.
- Segmentation: Deep learning algorithms (U-Net architectures) are trained to identify mitochondrial clusters based on the spatial texture of the FAD signal.
- Morphometrics: The system quantifies mitochondrial organization (clustering vs. diffuse distribution). In aging oocytes, mitochondria often aggregate abnormally. METAPHOR’s AI detects these subtle textural changes that escape the human eye, correlating mitochondrial morphology with the spectral metabolic readout.
4. Integration with Clinical Workflow
The proposed clinical prototype is an “add-on” module or a standalone benchtop unit. The embryo is briefly removed from the culture incubator (or imaged inside a time-lapse system equipped with 2PE ports), scanned (< 5 minutes), and returned. The AI generates a "Metabolic Score" (0-10) enabling the embryologist to rank order embryos for transfer.
Economic Impact Analysis for ART Centers
The adoption of METAPHOR involves significant CAPEX (likely $150k–$300k given the 2PE laser costs), but the OPEX and ROI arguments are compelling for high-volume clinics.
1. Reducing “Time to Pregnancy”
The primary driver of IVF cost and patient churn is the failed cycle.
- Current State: A patient may undergo 3 single-embryo transfers (SET) to achieve one live birth. Each failed transfer costs ~$3,000–$5,000 in thawing and procedure fees, plus emotional attrition.
- METAPHOR Impact: By selecting the metabolically competent embryo on the first attempt, the clinic increases the implantation rate per transfer. Reducing the average transfers-to-birth from 3.0 to 1.5 doubles the effective clinic throughput and significantly boosts patient satisfaction.
2. Displacing PGT-A Costs
While METAPHOR assesses metabolism, not genetics, strong correlations exist between metabolic chaos and aneuploidy. If METAPHOR can identify viable embryos with high sensitivity, it may serve as a triage tool, reducing the need for PGT-A in younger patients or acting as a superior non-invasive alternative for patients with few embryos who cannot risk biopsy damage.
- Cost Savings: Eliminating biopsy ($500/embryo) and reference lab fees ($200/embryo) saves the patient ~$2,000 per cycle, which can be re-allocated to the METAPHOR scan fee (premium add-on).
3. Premium Service Model
Clinics can bill METAPHOR as a “Metabolic Health Check” add-on.
- Scenario: 500 cycles/year.
- Fee: $500 per cohort scan.
- Revenue: $250,000/year.
- ROI: The device pays for itself in 12-18 months, purely on add-on revenue, excluding the efficiency gains from higher success rates.
Future Regulatory Path
1. Safety Profiles & Phototoxicity
The greatest hurdle for FDA De Novo classification or CE Marking is proving phototoxicity safety.
- Thermal Limits: The two-photon laser must not heat the culture media >0.5°C.
- ROS Generation: Exciting fluorophores can generate Reactive Oxygen Species (ROS). Validation studies (mouse/bovine) must prove that scanned embryos have identical blastulation and live-birth rates compared to unscanned controls. The PNAS 2024 study provides this foundational safety data, showing no detriment to murine embryo development.
2. Software as a Medical Device (SaMD)
The AI component falls under SaMD regulations.
- Transparency: The “black box” nature of Deep Learning is a regulatory challenge. The “Phasor” approach is advantageous here because it is based on explicit physics (Fourier transforms of spectra), making the feature extraction transparent/explainable compared to pure “end-to-end” CNNs.
- Generalizability: Algorithms trained on one incubator system (e.g., EmbryoScope) must be validated on others to ensure spectral calibration holds across different optical environments.
3. FDA Strategy
- De Novo Request: Since no predicate device exists for “hyperspectral metabolic embryo profiling,” this will likely be a Class II De Novo submission.
- Endpoints: The FDA will likely require a non-inferiority safety trial followed by a prospective efficacy trial showing correlation with implantation rates.
Conclusion
METAPHOR stands at the vanguard of the “Functional Embryology” era. For decades, IVF has relied on structural proxies to guess at physiological competence. By making the invisible visible—quantifying the molecular respiration of the embryo through non-invasive hyperspectral analysis—we are moving from subjective art to quantitative science.
For the IVF clinic, this technology offers a dual value proposition: a potent new revenue stream and a decisive tool to improve success rates. As the technology matures from the optical bench to the clinical workstation, it promises to answer the oldest question in the fertility lab with unprecedented precision: “Which one of these will become a baby?”
