If you’re planning to use artificial insemination (AI) as a means of achieving pregnancy, it’s important to understand the various methods of sperm preparation that are available. In this article, we’ll cover three main techniques: sperm washing, swim up, and density gradient centrifugation.
Sperm washing involves separating sperm from other fluids and substances in the semen sample. This can be done using a variety of methods, including centrifugation and filtration. The goal of sperm washing is to isolate the healthiest, most motile sperm in order to increase the chances of fertilization.
The swim up method involves placing a sample of semen in a culture medium and allowing the sperm to “swim” towards the surface. As the sperm rise to the top, they can be collected and used for AI. This method is thought to be effective because it allows the sperm to select their own path, potentially leading to a higher concentration of healthy sperm.
Density gradient centrifugation involves separating sperm from other cells and substances in the semen sample using a solution with a gradient of densities. The sperm are placed in the solution and then spun in a centrifuge, causing them to separate based on their density. The resulting sample contains a higher concentration of healthy sperm, which can be used for AI.
Overall, sperm preparation techniques can play a significant role in the success of AI. It’s important to discuss your options with a healthcare provider and choose the method that is most appropriate for your situation.
Introduction
Sperm cells, also known as spermatozoa, are incredibly complex and specialized cells with various regions and functions. Each sperm cell has a membrane made up of different types of lipids, such as phospholipids and glycolipids, as well as steroids. This membrane is divided into five main regions: the acrosomal region, the equatorial region, the post acrosomal region, the midpiece, and the tail.
As sperm cells move through the reproductive tract, their membranes undergo various changes in order to mature and prepare for fertilization. These changes, which can be specific to certain regions of the membrane, are thought to be crucial for the successful fertilization of the egg cell. Understanding the complexity and specialized functions of sperm cells can help researchers and healthcare professionals better understand and treat male infertility.
As our understanding of sperm cells and their role in fertility has grown, there has been an increased focus on developing effective methods for separating and preparing sperm samples. A good sperm preparation technique can help to ensure that the sample has a high percentage of viable, motile sperm, as well as considering other factors such as sperm capacitation and apoptotic state. These considerations are important because they can impact the sperm’s ability to fertilize an egg cell. By using advanced techniques for preparing sperm samples, reproductive medicine experts can help to improve the chances of successful fertilization and embryogenesis.
When it comes to processing sperm samples, it’s important to use techniques that are gentle and able to recover a high percentage of functional sperm. Some methods, such as serial centrifugation, can damage sperm cells and reduce their functionality. To avoid this, more gentle techniques such as double density gradient centrifugation and swim-up procedures have been developed and are now widely used in clinical practice. These methods allow for the selection of healthy, viable sperm without causing unnecessary harm or damage. Overall, the goal of sperm processing is to obtain a sample with a high concentration of functional sperm that are capable of fertilising an egg cell, and gentle techniques are key to achieving this goal.
If you and your partner are having trouble conceiving, it might be helpful to try sperm washing as a way to increase your chances of success. Sperm washing is a process that involves separating sperm cells from other substances in the semen sample. It’s often used in conjunction with artificial insemination (AI), a type of assisted reproductive technique.
One reason sperm washing is helpful is because some components of the seminal fluid can interfere with fertilisation when AI or in vitro fertilisation (IVF) is performed. Sperm cells and leukocytes (white blood cells) can produce oxygen radicals that can decrease the likelihood of sperm-egg fusion after multiple rounds of centrifugation. By using methods like swim-up or gradient density centrifugation to carefully select the sperm cells, it’s possible to avoid these negative effects and increase the chances of successful fertilisation.
In recent years, researchers have been working to develop more sophisticated techniques for separating functional sperm cells from those that are not viable for fertilisation. Intrauterine insemination (IUI) and ovarian stimulation are still commonly used treatments for male subfertility. There are several methods available for preparing sperm for artificial insemination, and the best one to use will depend on the quality of the semen sample, including factors such as concentration, motility, and morphology. These techniques include migration methods, density gradient centrifugation, and filtration methods.
When comparing the effectiveness of various semen processing methods, researchers have found that several key factors can indicate the quality of the sperm cells obtained. These include viability, motility, and morphology. A higher concentration of viable, motile sperm cells with normal morphology increases the chances of successful fertilisation. By using techniques that consider these factors, it’s possible to obtain a sample of high-quality sperm cells that are more likely to result in a successful pregnancy.
Sperm preparation procedures refer to methods used to isolate and prepare sperm for use in assisted reproductive technologies such as in vitro fertilisation (IVF) or artificial insemination. Pre-wash procedures are steps taken to clean and prepare sperm for further processing.
Here are some common pre-wash procedures:
- Sperm washing: This involves separating sperm from other components of the semen sample, such as prostate and seminal vesicle secretions, using a centrifuge. The resulting sperm pellet is resuspended in a medium that supports sperm motility and viability.
- Sperm selection: This involves using techniques such as swim-up or density gradient separation to select motile sperm for use in assisted reproduction.
- Sperm preparation for cryopreservation: If the sperm will be frozen for later use, it must be prepared and stored in a cryoprotectant solution to prevent damage during the freezing process.
It is important to follow proper sperm preparation procedures to ensure that the sperm used in assisted reproduction are of high quality and able to fertilise an egg successfully.
1.2. FOR DIAGNOSTIC OR RESEARCH PURPOSES, A COLLECTION OF SEMEN IS NEEDED.
In order to obtain a high-quality semen sample for diagnostic or research purposes, it is important to follow certain guidelines for collection and handling of the sample. The sample should be collected in a private room near the laboratory to limit exposure to temperature fluctuations and to ensure that the sample can be promptly processed.
The specimen container should be placed on a bench or in an incubator set at 37°C to allow the semen to liquefy. The container should be kept at a consistent temperature between 20°C and 37°C to avoid temperature changes that could affect the sperm. It is important to label the container with the man’s name and identification number, as well as the date and time of collection.
- BASIC SEMEN TESTING, STANDARD SEMEN FLUID TESTS, AND SPECIFICALLY DESIGNED SEMEN TESTS FOR DIAGNOSTIC OR RESEARCH PURPOSES ARE THE SPERM PREPARATION PROCEDURES [PRE–WASH PROCEDURES].
Semen analysis is a important step in the evaluation of male fertility. It involves examining the physical and chemical properties of a man’s semen sample to identify any problems that may affect the quality of the semen. This can help experts determine the best course of treatment or intervention to improve the chances of a successful pregnancy. Semen analysis can also help to identify underlying medical issues that may be contributing to poor results. By identifying and addressing any problems with the semen, it may be possible to reduce the need for more complex interventions for the female partner.
FOR DIAGNOSTIC OR RESEARCH PURPOSES, THE BASIC SEMEN TESTING
That’s correct. Semen is the fluid that is ejaculated during male orgasm and contains sperm cells as well as secretions from the prostate and seminal vesicles. Semen analysis involves examining a freshly ejaculated semen sample under a microscope to assess the number, shape, and movement of sperm. It is a vital part of diagnosing male infertility and should be performed at a specialised laboratory using methods approved by the World Health Organization (WHO). Semen analysis can help determine whether there is a male factor contributing to a couple’s subfertility and can also identify potential problems, such as infection or inflammation, based on certain findings. However, it is important to note that some abnormalities in the semen analysis parameters may be non-specific and may have multiple causes, some of which may have serious medical implications. A thorough evaluation can help determine the cause of an abnormal semen analysis.
2.1. The first macroscopic semen examination is performed
Semen analysis should begin as soon as possible after ejaculation, preferably within 30 minutes but no later than 1 hour to avoid changes in temperature or dehydration that could affect the quality of the sample. The semen sample should be examined under a microscope to assess the number, shape, and movement of the sperm. This information can help to identify any problems with the sperm or the surrounding fluid that may affect fertility. Semen analysis is an important part of diagnosing male infertility and should be performed at a specialised laboratory using methods approved by the World Health Organization (WHO).
2.1.1. What is Semen liquefaction?
After ejaculation, semen is typically a semisolid coagulated mass. Within a few minutes at room temperature, the semen usually begins to liquefy and becomes more homogeneous. This process is known as liquefaction and is important for semen analysis because it allows the sperm to move more freely within the fluid. Liquefaction usually occurs within 15 minutes at room temperature, although it can take up to 60 minutes in some cases. If complete liquefaction does not occur within 60 minutes, this should be recorded. During the process of liquefaction, small jelly-like granules known as gelatinous bodies may be present in the fluid. These do not affect semen quality and do not need to be recorded. However, the presence of mucus strands may interfere with semen analysis and should be noted.
2.1.2. Semen viscosity
Semen viscosity is a measure of the thickness or stickiness of the semen. It can be evaluated by gently aspirating the semen into a wide-bore plastic pipette and observing the length of the thread that forms as the semen drops by gravity. A normal sample will form small discrete drops, while an abnormal sample will form a thread more than 2 cm long. Alternatively, the viscosity can be evaluated by introducing a glass rod into the sample and observing the length of the thread that forms upon withdrawal of the rod. High viscosity can interfere with certain aspects of semen analysis, such as the determination of sperm motility, concentration, and the detection of antibody-coated sperm. It is important to note that high viscosity can be recognized by the elastic properties of the sample, which will adhere strongly to itself when attempts are made to pipette it.
2.1.3. Appearance of the liquefied semen [ejaculate]
A normal liquefied semen sample has a homogeneous, grey-opalescent appearance. The appearance of the sample may be affected by certain factors, such as the sperm concentration, the presence of red blood cells (haemospermia), or the presence of certain substances in the body, such as bilirubin in cases of jaundice or certain vitamins or drugs. A normal semen sample may appear less opaque if the sperm concentration is very low, and may have a different color, such as red-brown in the presence of red blood cells or yellow in the presence of certain substances. It is important to note that changes in the appearance of the semen sample may indicate underlying health issues or other problems that may affect fertility.
2.1.4. Semen volume
The volume of the liquefied semen is an important factor to consider in semen analysis because it allows the total number of sperm cells and non-sperm cells in the ejaculate to be calculated. The volume of the semen is contributed mainly by the seminal vesicles and prostate gland, with a smaller contribution from the bulbourethral glands and epididymides. Low semen volume can be a characteristic of certain conditions, such as obstruction of the ejaculatory duct or congenital bilateral absence of the vas deferens, which can also lead to poorly developed seminal vesicles. Low semen volume can also be the result of collection problems, partial retrograde ejaculation, or androgen deficiency. On the other hand, high semen volume may reflect active exudation in cases of active inflammation of the accessory organs.
2.1.5. Semen pH
The pH of semen reflects the balance between the pH values of the secretions produced by the accessory glands, including the alkaline seminal vesicular secretion and the acidic prostatic secretion. The pH of the semen should be measured after liquefaction, preferably within 30 minutes but no later than 1 hour after ejaculation, as it can be affected by the loss of CO2 that occurs after production. A pH value less than 7.0 in a semen sample with low volume and low sperm numbers may indicate ejaculatory duct obstruction or congenital bilateral absence of the vas deferens, which can also lead to poorly developed seminal vesicles. Abnormal pH values can affect sperm function and may be an indication of underlying health issues or other problems that may affect fertility.
2.2. Initial microscopic semen examination
An initial microscopic examination of the semen sample involves scanning the preparation to obtain an overview of the sample and identify any abnormalities. During this process, the presence of mucus strands, sperm aggregation or agglutination, and cells other than sperm cells, such as epithelial cells, leukocytes, or immature germ cells, may be observed. The examination also involves assessing sperm motility and determining the dilution required for accurate assessment of sperm number. This information is important for identifying any problems with the sperm or the surrounding fluid that may affect fertility.
2.2.1. Thorough mixing and representative sampling of semen
The nature of the liquefied ejaculate can make it difficult to obtain a representative sample of semen for analysis. If the sample is not well mixed, analysis of two separate aliquots may reveal significant differences in sperm motility, vitality, concentration, and morphology. This can make it challenging to accurately assess the quality of the semen and identify any problems that may affect fertility. It is important to mix the semen sample thoroughly and take care to obtain a representative sample for analysis to ensure the most accurate results.
2.2.2. Aggregation of spermatozoa
The adherence of immotile sperm cells to each other or of motile sperm cells to mucus strands, non-sperm cells, or debris is known as nonspecific aggregation. It is considered to be a nonspecific abnormality and should be recorded as such during semen analysis. Nonspecific aggregation can interfere with the movement and function of the sperm cells and may affect fertility. It is important to note and record any instances of nonspecific aggregation during semen analysis to help identify potential problems that may affect fertility.
2.2.3. Agglutination of spermatozoa
Agglutination specifically refers to the sticking together of motile sperm cells in various ways, such as head-to-head, tail-to-tail, tail-tip-to-tail-tip, or in a mixed way. Agglutination can affect the movement and function of the sperm cells and may interfere with fertility. The degree and site of attachment of the agglutinates can be graded from 1 to 4, with grade 1 representing isolated agglutinates with fewer than 10 sperm cells per agglutinate and many free sperm cells, grade 2 representing moderate agglutinates with 10-50 sperm cells per agglutinate and free sperm cells, grade 3 representing large agglutinates with more than 50 sperm cells per agglutinate and some free sperm cells, and grade 4 representing gross agglutination with all sperm cells agglutinated and interconnected agglutinates. The presence of agglutination may be suggestive of the presence of anti-sperm antibodies, but further testing is required to confirm this. Severe agglutination can affect the assessment of sperm motility and concentration. It is important to note and record any instances of agglutination during semen analysis to help identify potential problems that may affect fertility.
2.2.4. Cellular elements other than spermatozoa
The ejaculate contains cells other than sperm cells, some of which may be clinically relevant. These cells include epithelial cells from the genitourinary tract, leukocytes, and immature germ cells. Leukocytes and immature germ cells are often referred to collectively as “round cells.” The presence of these cells in the ejaculate can be an indication of underlying health issues or other problems that may affect fertility. It is important to note and record the presence of any cells other than sperm cells during semen analysis to help identify potential issues that may need to be addressed.
2.2.5. Sperm motility (sometimes referred to as “mobility”):Sperm motility refers to the percentage of sperm that are moving. In even the best specimens, many sperm are not moving and may be either dead or simply not moving at the time of analysis. Motility is typically measured as a percentage, with 100% indicating that all the sperm are moving and 0% indicating that no sperm are moving. Most specimens fall somewhere in between these extremes. To measure sperm motility, the sperm are placed on an etched slide and the number of moving and immotile (non-moving) sperm are counted in numerous boxes in the grids. The percentage of moving sperm is then calculated by dividing the total number of moving sperm by the total number of sperm. It is important to note that sperm motility can be affected by issues with production as well as issues that occur within the ducts that store and transport the sperm, such as infections. Normal sperm motility is considered to be 50% or more of the moving sperm. Low sperm motility can be a factor in fertility problems and should be carefully evaluated.
2.2.5.1. Categories of sperm motility [sperm movement]
The process of semen analysis is an essential part of diagnosing male infertility. It involves laboratory testing of freshly ejaculated semen to assess the number, shape, and movement of sperm. A normal semen sample is expected to have a homogeneous, grey-opalescent appearance and complete liquefaction within 15 minutes at room temperature. The volume of the liquefied semen is an important factor in calculating the total number of sperm and non-sperm cells present in the ejaculate. A pH value of less than 7.0 may indicate ejaculatory duct obstruction or congenital bilateral absence of the vas deferens.
A microscopic examination of the semen sample is conducted to check for factors such as mucus strand formation, sperm aggregation or agglutination, and the presence of cells other than spermatozoa. Sperm motility is also assessed and graded on a scale of progressive, non-progressive, or immotile. Forward progression, or the quality of movement of the sperm, is also evaluated on a scale of 0-4. The total motile count (TMC), or the number of moving sperm in the entire ejaculate, is calculated by multiplying the volume, concentration, and motility. A TMC of over 10 million is considered a reasonable chance for success with intrauterine insemination (IUI), while a TMC less than 10 million may indicate the need for in vitro fertilization (IVF).
2.2.6. Sperm vitality:
Sperm viability is an important factor in fertility and a semen analysis is used to evaluate the number of living sperm in a man’s ejaculate. There are several methods that can be used to determine sperm viability, including the dye exclusion method and the hypo-osmotic swelling test. The dye exclusion method involves staining the sperm with a dye that is not able to enter cells with intact membranes. The hypo-osmotic swelling test involves exposing the sperm to a hypotonic solution, which will cause cells with intact membranes to swell. A normal sperm sample should have a viability of at least 40%. If the viability is lower than this, it may be an indication of a fertility issue. It is important to assess sperm viability as soon as possible after ejaculation to prevent any changes in temperature or dehydration from affecting the results.
2.2.7. Sperm numbers
Sperm concentration is usually measured in millions of sperm per milliliter (mL) of semen. A normal sperm concentration is considered to be at least 15 million sperm per mL of semen, or at least 39 million sperm per ejaculate. However, it is important to note that sperm concentration can vary greatly among individuals and can be affected by a number of factors, including age, lifestyle, and overall health.
While a high sperm concentration can increase the chances of fertility, it is not the only factor to consider. The motility (movement) and viability (health) of the sperm are also important in determining fertility potential. A sperm sample with a high concentration but low motility or viability may not be as effective at fertilizing an egg as a sample with a lower concentration but higher motility and viability.
Sperm concentration can be measured through a process called sperm count. This involves collecting a sample of semen and analyzing it under a microscope to count the number of sperm present. Sperm count is usually done as part of a semen analysis, which is a laboratory test that evaluates the quality and quantity of a man’s sperm. A semen analysis is often used to diagnose male infertility and to determine the underlying cause of fertility issues in men. It can also be used to monitor the effectiveness of treatment for fertility problems.
Concentration (sometimes referred to as “the count”):It is important to note that sperm concentration, motility, and total motile count are all important factors in fertility. Low sperm concentration, or a low percentage of motile sperm, can decrease the chances of fertilization, as there may not be enough sperm present or capable of moving to successfully reach and fertilize an egg. Total motile count, which takes into account both concentration and motility, is often used to determine the likelihood of successful fertilization through methods such as intrauterine insemination (IUI) or in vitro fertilization (IVF). A TMC of over 10 million is generally considered necessary for a reasonable chance of success with IUI, while a TMC of less than 10 million may indicate the need for IVF. It is also important to note that forward progression, or the quality of sperm movement, is crucial for successful fertilization, as sperm must be able to move effectively towards the egg in order to fertilize it.
2.2.7. Normal sperm morphology
Normal sperm morphology refers to the shape of the sperm, and specifically, the criteria that the sperm must meet to be considered normal. According to the World Health Organization (WHO) criteria, normal sperm should have an oval head, a long and slender midsection, and a long tail. However, it’s important to note that the WHO criteria are not perfect and may not be as detailed as other methods for evaluating sperm morphology. It’s also important to note that sperm morphology is just one aspect of sperm health, and other factors such as motility (the ability of the sperm to move properly) and concentration also play a role in fertility. If you have concerns about your sperm health or fertility, it’s important to speak with a healthcare provider or fertility specialist.
The concept of morphologically normal spermatozoa
Morphologically normal spermatozoa refers to sperm that have a normal shape according to the criteria set by the World Health Organization (WHO). In order to be considered morphologically normal, sperm should have an oval head, a long and slender midsection, and a long tail. These criteria are used by most commercial laboratories to evaluate sperm morphology. However, it’s important to note that the WHO criteria are not perfect and may not be as detailed as other methods for evaluating sperm shape. For example, some specialty labs may use a more sophisticated method called Strict, Kruger, or Tygerberg Morphology, which is considered to be more accurate but is also more time-consuming to perform. It’s also important to note that sperm morphology is just one aspect of sperm health, and other factors such as motility (the ability of the sperm to move properly) and concentration also play a role in fertility. If you have concerns about your sperm health or fertility, it’s important to speak with a healthcare provider or fertility specialist.
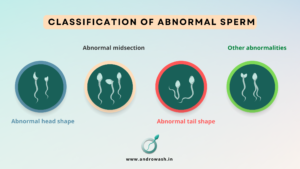
2.2.8. Classification of abnormal sperm morphology
There are several different categories of abnormal sperm morphology, which refer to deviations from the criteria for normal sperm shape set by the World Health Organization (WHO). Some common categories of abnormal sperm morphology include:
- Abnormal head shape: This includes sperm with an abnormally large or small head, or sperm with a misshapen or asymmetrical head.
- Abnormal midsection: This includes sperm with a midsection that is too thin or too wide, or sperm with a midsection that is bent or curved.
- Abnormal tail shape: This includes sperm with a short, thick, or double tail, or sperm with a tail that is bent or coiled.
- Other abnormalities: This includes sperm with multiple heads or tails, or sperm with structural defects such as holes or ridges.
It’s important to note that abnormal sperm morphology can be caused by a variety of factors, including genetics, infection, and exposure to certain toxins or medications. If you have concerns about your sperm health or fertility, it’s important to speak with a healthcare provider or fertility specialist.
2.2.9. Indices of multiple sperm defects
There are several indices that can be used to measure the extent of multiple sperm defects, or the presence of multiple abnormalities in the shape of the sperm. Some common indices for measuring multiple sperm defects include:
- Percent normal forms (PNF): This index measures the percentage of sperm that have a normal shape according to the criteria set by the World Health Organization (WHO). Sperm with abnormal head shape, midsection, or tail shape are not considered normal forms.
- Kruger strict criteria: This index is a more detailed and specific way of evaluating sperm shape that is used by some specialty labs. It takes into account a variety of characteristics, including head shape, midsection, and tail shape. Sperm that do not meet the criteria for each characteristic are considered defects.
- Sperm defect index (SDI): This index measures the percentage of sperm with defects according to the Kruger strict criteria. It is calculated by dividing the total number of defects by the total number of sperm counted, and multiplying by 100.
- Head defects index (HDI): This index measures the percentage of sperm with head defects according to the Kruger strict criteria. It is calculated by dividing the total number of head defects by the total number of sperm counted, and multiplying by 100.
It’s important to note that these indices are just a few examples, and other methods may also be used to measure multiple sperm defects. If you have concerns about your sperm health or fertility, it’s important to speak with a healthcare provider or fertility specialist.
2.2.10. Assessment of specific sperm defects
There are several methods that can be used to assess specific sperm defects, or abnormalities in the shape of the sperm. Some common methods for evaluating specific sperm defects include:
- Microscopy: Sperm defects can be identified using a microscope, which allows for detailed visualization of the sperm’s shape and structure. This can be done using either light microscopy or electron microscopy, depending on the level of detail needed.
- Staining: Sperm defects can also be identified by staining the sperm with a special dye that highlights specific structures or abnormalities. This can be done using a variety of stains, such as hematoxylin and eosin (H&E) or Papanicolaou (PAP) stain.
- Computer-assisted sperm analysis (CASA): This method uses a computer and specialized software to analyze the shape and movement of the sperm. It can be used to identify specific defects such as abnormal head shape or abnormal tail shape.
- Other methods: There are many other methods that can be used to assess specific sperm defects, including flow cytometry, immunofluorescence microscopy, and DNA fragmentation analysis.
It’s important to note that different methods may be more suitable for evaluating certain types of defects, and that multiple methods may be used in combination to get a complete understanding of the sperm’s shape and structure. If you have concerns about your sperm health or fertility, it’s important to speak with a healthcare provider or fertility specialist.
2.2.11. Testing for antibody coating of spermatozoa
There are several methods that can be used to test for the presence of antibodies coating the spermatozoa, or the reproductive cells in men. Some common methods for testing for antibody coating of spermatozoa include:
- Sperm penetration assay (SPA): This method involves incubating the sperm with cervical mucus or other substances that mimic the female reproductive tract. The sperm’s ability to penetrate these substances is then observed to determine if there are any antibodies present that may be hindering their movement.
- Sperm binding test: This test involves incubating the sperm with a substance that binds to any antibodies present on the sperm surface. The amount of bound substance is then measured to determine the presence and level of antibodies.
- Immunobead test: This test involves adding small beads coated with an antibody to the sperm sample. If any antibodies are present on the sperm surface, they will bind to the beads. The presence of bound beads can then be detected using a microscope or other imaging technique.
- Immunofluorescence assay (IFA): This test involves adding a fluorescent dye to the sperm sample and then observing the sperm under a microscope. If any antibodies are present on the sperm surface, they will fluoresce, allowing them to be detected.
It’s important to note that these are just a few examples of methods that can be used to test for the presence of antibody coating on spermatozoa, and that other methods may also be available. If you have concerns about your sperm health or fertility, it’s important to speak with a healthcare provider or fertility specialist.
STANDARD SEMEN FLUID TESTS FOR DIAGNOSTIC PURPOSES OR RESEARCH PURPOSES
There are several standard semen fluid tests that are commonly used for diagnostic or research purposes. Some common tests include:
- Sperm concentration: This test measures the number of sperm per milliliter of semen. A normal sperm concentration is typically at least 15 million sperm per milliliter.
- Sperm motility: This test measures the percentage of sperm that are moving properly and the quality of their movement. Normal sperm motility is typically at least 40% for progressive motility (forward movement) and at least 32% for total motility (any movement).
- Sperm morphology: This test evaluates the shape of the sperm according to the criteria set by the World Health Organization (WHO). Normal sperm morphology is typically at least 4% according to the WHO criteria.
- Semen pH: This test measures the acidity or alkalinity of the semen. A normal semen pH is typically between 7.2 and 7.8.
- Semen volume: This test measures the amount of semen produced during ejaculation. A normal semen volume is typically at least 1.5 milliliters.
- Semen fructose: This test measures the presence and amount of fructose in the semen. Fructose is a sugar that is produced by the prostate gland and is used as an energy source for the sperm.
These are just a few examples of standard semen fluid tests that are commonly used for diagnostic or research purposes. Other tests may also be available, and the specific tests used may vary depending on the purpose of the evaluation and the specific concerns or issues being addressed. If you have concerns about your sperm health or fertility, it’s important to speak with a healthcare provider or fertility specialist.
SPECIALIZED SEMEN TESTS FOR DIAGNOSTIC PURPOSES OR RESEARCH PURPOSES
There are several specialized semen tests that may be used for diagnostic or research purposes in addition to or instead of the standard semen fluid tests. Some examples of specialized semen tests include:
- Sperm DNA fragmentation: This test measures the percentage of sperm with damaged DNA. High levels of DNA fragmentation can negatively impact fertility.
- Sperm aneuploidy testing: This test looks for chromosomal abnormalities in the sperm. Aneuploidy (an abnormal number of chromosomes) can cause fertility problems or pregnancy complications.
- Antisperm antibodies: This test looks for the presence of antibodies that may be attacking or blocking the sperm. Antisperm antibodies can affect fertility by hindering the sperm’s movement or ability to fertilize an egg.
- Sperm function tests: These tests evaluate the sperm’s ability to perform various functions, such as binding to the egg or penetrating the egg’s outer layers.
- Sperm chromosomal abnormalities: This test looks for chromosomal abnormalities in the sperm that may be associated with fertility problems or pregnancy complications.
- Sperm proteome analysis: This test looks at the proteins present in the sperm and how they are expressed. This can provide information about the sperm’s function and potential fertility.
These are just a few examples of specialized semen tests that may be used for diagnostic or research purposes. Other tests may also be available, and the specific tests used may vary depending on the purpose of the evaluation and the specific concerns or issues being addressed. If you have concerns about your sperm health or fertility, it’s important to speak with a healthcare provider or fertility specialist.
ADDITIONAL SPERM–OOCYTE INTERACTION TESTS
There are several additional tests that can be used to evaluate the interaction between sperm and oocytes (eggs) in order to assess fertility. Some examples of sperm-oocyte interaction tests include:
- In vitro fertilization (IVF): This is a laboratory procedure in which sperm and eggs are combined outside the body in a dish, and the fertilized egg (embryo) is then transferred to the woman’s uterus. IVF can be used to evaluate sperm-oocyte interaction and assess fertility.
- Intracytoplasmic sperm injection (ICSI): This is a procedure in which a single sperm is injected directly into an egg in order to fertilize it. ICSI is often used in cases of severe male infertility or when other fertilization methods have failed.
- Sperm-oocyte co-culture: This test involves culturing sperm and eggs together in a dish to observe their interaction and assess fertility.
- Sperm-oocyte adhesion assay: This test measures the ability of the sperm to attach to the egg’s outer layers (cumulus cells) and penetrate through them.
- Zona pellucida binding assay: This test measures the ability of the sperm to bind to the egg’s outer layers (zona pellucida).
These are just a few examples of tests that can be used to evaluate the interaction between sperm and oocytes. Other tests may also be available, and the specific tests used may vary depending on the purpose of the evaluation and the specific concerns or issues being addressed. If you have concerns about your sperm health or fertility, it’s important to speak with a healthcare provider or fertility specialist.
- SPERM WASHING AS THE PREPARATIONAL PROCESS FOR PERFORMING AN INTRAUTERINE INSEMINATION (IUI)
Sperm washing is a process used to prepare sperm for use in an intrauterine insemination (IUI) procedure. IUI is a fertility treatment in which sperm are inserted directly into the woman’s uterus in order to increase the chances of fertilization. Sperm washing is used to separate the sperm from the other components of the semen, such as the seminal fluid, in order to increase the concentration of sperm and improve the chances of fertilization.
There are several steps involved in the sperm washing process:
- Semen collection: The semen is collected using masturbation or a special condom that is worn during intercourse.
- Semen processing: The collected semen is processed in a laboratory setting using a special solution or centrifuge to separate the sperm from the other components of the semen.
- Sperm preparation: The separated sperm are then prepared for the IUI procedure by being placed in a special medium or washed with a solution to remove any remaining debris.
- IUI: The prepared sperm are then inserted into the woman’s uterus using a thin, flexible tube (catheter).
Sperm washing is typically performed by a fertility specialist or trained laboratory technician in a sterile environment. If you are considering an IUI procedure and have questions about sperm washing or other aspects of the process, it’s important to speak with a healthcare provider or
3.1. When spermatozoa may need to be separated from seminal plasma
Spermatozoa may need to be separated from seminal plasma, or the fluid portion of the semen, in a number of situations. Some examples include:
- Intrauterine insemination (IUI): Sperm washing is often used to prepare sperm for use in IUI, a fertility treatment in which sperm are inserted directly into the woman’s uterus. Separating the sperm from the seminal plasma can increase the concentration of sperm and improve the chances of fertilization.
- Assisted reproductive technologies (ART): In ART procedures such as in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), sperm may need to be separated from the seminal plasma in order to be used in the laboratory setting.
- Donor sperm: Sperm from a sperm donor may need to be separated from the seminal plasma in order to be used in fertility treatments or for artificial insemination.
- Research: Sperm may need to be separated from the seminal plasma for research purposes, such as to study the effects of different components of the semen on fertility.
It’s important to note that sperm washing is typically performed by a fertility specialist or trained laboratory technician in a sterile environment. If you have questions about sperm washing or other aspects of fertility treatment, it’s important to speak with a healthcare provider or fertility specialist.
3.2. The choice of sperm preparation technique
The choice of sperm preparation technique, or the method used to separate the sperm from the other components of the semen, can depend on a number of factors. Some of the considerations that may influence the choice of sperm preparation technique include:
- The intended use of the prepared sperm: Different techniques may be more suitable for different purposes, such as IUI, IVF, or artificial insemination.
- The quality of the semen: The technique used may depend on the overall quality of the semen and the concentration of sperm present.
- The presence of any abnormalities or infections: Certain techniques may be more suitable for sperm with certain types of abnormalities or if there is a risk of infection.
- The preference of the fertility specialist or patient: Different techniques may have different benefits and drawbacks, and the fertility specialist or patient may have a preference for one technique over another.
It’s important to note that the choice of sperm preparation technique may vary depending on the specific situation and the goals of the treatment. If you have questions about sperm preparation or other aspects of fertility treatment, it’s important to speak with a healthcare provider or fertility specialist.
3.3. Efficiency of sperm separation from seminal plasma and infectious organisms
The efficiency of sperm separation from seminal plasma and infectious organisms, or the ability to effectively separate the sperm from the other components of the semen and any potentially harmful microorganisms, can vary depending on the technique used and the specific characteristics of the semen. Some sperm preparation techniques may be more efficient at separating the sperm from the seminal plasma and infectious organisms than others.
For example, the swim-up technique is a commonly used method for separating sperm from the seminal plasma and infectious organisms. In this technique, the semen is placed in a special medium and allowed to “swim up” to the surface, where the sperm can be collected. This technique can be efficient at separating the sperm from the seminal plasma and infectious organisms, but it may not be suitable for all situations.
Other techniques that may be used to separate sperm from the seminal plasma and infectious organisms include density gradient centrifugation, which uses a special solution to separate the sperm based on their density, and swim-down, which is similar to the swim-up technique but involves collecting the sperm from the bottom of the medium rather than the top.
It’s important to note that the efficiency of sperm separation from seminal plasma and infectious organisms may vary depending on the technique used and the specific characteristics of the semen. If you have questions about sperm preparation or other aspects of fertility treatment, it’s important to speak with a healthcare provider or fertility specialist.
3.4. General principles of sperm preparation techniques
There are several general principles that apply to sperm preparation techniques, or methods used to separate the sperm from the other components of the semen. These principles include:
- Asepsis: Sperm preparation techniques should be performed in a sterile environment to prevent contamination with infectious organisms.
- Sperm viability: Techniques should be designed to minimize damage to the sperm and maximize their viability, or ability to fertilize an egg.
- Sperm concentration: Techniques should aim to increase the concentration of sperm in the prepared sample in order to improve the chances of fertilization.
- Specificity: Techniques should be specific to the sperm and avoid contaminating the prepared sample with other cells or substances.
- Efficiency: Techniques should be efficient at separating the sperm from the seminal plasma and other contaminants in order to maximize the yield of prepared sperm.
- Safety: Techniques should be safe for both the patient and the laboratory personnel.
It’s important to note that these are just a few general principles that apply to sperm preparation techniques, and that specific techniques may have their own unique considerations. If you have questions about sperm preparation or other aspects of fertility treatment, it’s important to speak with a healthcare provider or fertility specialist.
What is the essence of sperm washing process?
The essence of the sperm washing process is to separate the sperm from the other components of the semen, such as the seminal fluid, in order to increase the concentration of sperm and improve the chances of fertilization. Sperm washing is often used to prepare sperm for use in fertility treatments such as intrauterine insemination (IUI) or assisted reproductive technologies (ART) such as in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI).

There are several steps involved in the sperm washing process:
- Semen collection: The semen is collected using masturbation or a special condom that is worn during intercourse.
- Semen processing: The collected semen is processed in a laboratory setting using a special solution or centrifuge to separate the sperm from the other components of the semen.
- Sperm preparation: The separated sperm are then prepared for the fertility treatment by being placed in a special medium or washed with a solution to remove any remaining debris.
- Fertility treatment: The prepared sperm are then used in the chosen fertility treatment, such as IUI or IVF.
Sperm washing is typically performed by a fertility specialist or trained laboratory technician in a sterile environment. If you have questions about sperm washing or other aspects of fertility treatment, it’s important to speak with a healthcare provider or fertility specialist.
What are the main techniques of sperm washing for intrauterine insemination (IUI)?
There are several techniques that can be used for sperm washing in preparation for an intrauterine insemination (IUI) procedure. Some common techniques include:
- Swim-up: This technique involves placing the semen in a special medium and allowing the sperm to “swim up” to the surface, where they can be collected.
- Density gradient centrifugation: This technique involves using a special solution to separate the sperm from the other components of the semen based on their density.
- Swim-down: This technique is similar to the swim-up technique, but involves collecting the sperm from the bottom of the medium rather than the top.
- Percoll gradient: This technique involves using a special solution called Percoll to separate the sperm based on their density.
It’s important to note that these are just a few examples of techniques that can be used for sperm washing in preparation for IUI, and that other techniques may also be available. The specific technique used may depend on the characteristics of the semen and the specific goals of the treatment. If you have questions about sperm washing or other aspects of fertility treatment, it’s important to speak with a healthcare provider or fertility specialist.
Conclusion
In summary, sperm processing techniques such as swim-up and density gradient centrifugation are commonly used to separate sperm from the seminal plasma and other contaminants in preparation for fertility treatments such as IVF and ICSI. These techniques can help to improve the quality and viability of the prepared sperm by eliminating immotile and dead sperm, exfoliated cells, and other debris. Swim-up is typically preferred for normozoospermic specimens with good motility, while density gradient centrifugation is often used for ejaculates with low sperm number, motility, or morphology. The specific technique used may depend on the characteristics of the semen and the specific goals of the treatment. If you have questions about sperm processing or other aspects of fertility treatment, it’s important to speak with a healthcare provider or fertility specialist.
